University of Pittsburgh lab shows phage attacks in new light
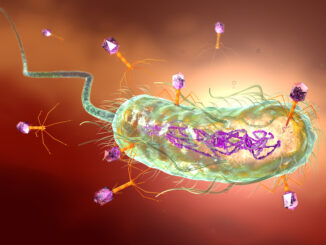
As antibacterial resistance continues to render obsolete the use of some antibiotics, some have turned to bacteria-killing viruses to treat acute infections as well as some chronic illnesses. Graham Hatfull, the Eberly Family Professor of […]