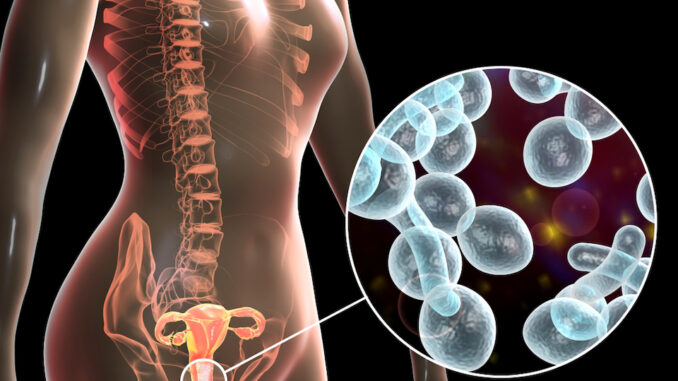
Proge Farm, an Italian pharmaceutical company, has made microbiome history with its recently approved therapeutic and will now be able to market its drug based on Lactobaccilus plantarum P 17630.
The company obtained authorization from the health authority for a new gynecological drug that can be marketed in the first group of EU countries. It is a vaginal topical in soft and hard capsules based on Lactobaccilus plantarum P 17630, produced in their Novara plant.
With an estimated 75 percent of women having vulvovaginal candidiasis at some time in life, the second leading cause of vaginitis, the intervention will no doubt be a welcome addition to healthcare provider’s therapeutic arsenal.
PROGE FARM was founded in 1992 as a company that mainly deals with consultancy in the area of regulatory affairs and pharmacovigilance. Today these activities are held by the associated company PROGE MEDICA.
In 1995 the company obtained the first registration of generic drugs which has been marketed through our own network of sales agents and in 2001 they opened their own research laboratory to select probiotic bacteria, inside the Technology Centre of Novara.
Since 2010, they produce their bacterial strains in its GMP manufacturing plant.

