
Introduction
The microbiome is one of the hottest areas in biotechnology, as countless studies have linked its composition and activity with human disease1,2, especially in illnesses of poorly understood mechanisms, with high medical unmet needs. Some authors have even proposed that the definition of communicable disease may have to be revisited after advances in microbiome science have shown some ailments believed to be noncommunicable may be transmitted through microbes3,4. This why it has been referred to as a forgotten organ5 which may thus be the missing link to better diagnose and potentially cure many diseases. This has made thousands of companies focus their attention on it.
Microbiome research has traditionally been performed by food companies developing probiotics and prebiotics. However, over the last years, and thanks to recent discoveries of how human disease and resident bacteria are interconnected, the field is also capturing attention from pharmaceutical companies which see the microbiome as a tool to develop new blockbuster drugs. This is why a number of biotechs, mostly startups, have launched microbiome-centric research programs.
The information employed to create this article was extracted from an analysis of the Microbiome Drug Database™, a comprehensive repository for pharmabiotic companies and research programs, powered by Sandwalk BioVentures and supported by The Microbiome Times. We scanned the landscape of biotechnology companies developing these and found a total of 203 entities which met our inclusion criteria (we excluded, amongst others, universities and research institutes; food or cosmetics companies; service providers like CMOs, CROs or sampling or sequencing companies; developers of non-highly-selective antimicrobials, vaccines and oncolytic viruses; as well as big pharmas with which the original microbiome drug developers have partnered). These companies we analyzed are collectively working on 650 active research programs. We additionally analyzed some key financials of these companies to have a full scientific, clinical, regulatory and economic view of the entire sector.
Key figures
A growing industry with a tipping point in 2014
The in-depth study of the human microbiome is a relatively new area of research, which only took off with the initiation of the Human Microbiome Project6 in 2007. Since then, the number of academic publications and patents on the microbiome has grown very significantly – at a higher pace since 2012. In fact, the 2010s have recently been nicknamed as the decade of the microbiome by Forbes7, as the most significant milestones in microbiota research have happened over the last 10 years8.
This better understanding of the link between the microbiome and disease, coupled with IP protection and a favorable economic and investment environment, led to an explosion of the number of microbiome biotechnology companies in 2014. That year alone saw the birth of 28 companies, and since then 60% of the companies have been founded.
A global € 4Bn bet, albeit, slowing down?
Microbiome therapeutics and diagnostics companies have collectively attracted a total of €4 Bn in investments to date. Most of these operations have also taken place in the 2014-2019 period, with over 80% of the 233 financial rounds recorded happening and 90% of the funds being committed in these last five years by almost 300 different investment companies.
Most of these pharmabiotic companies remain private and have completed only one or two funding rounds. Over 60% of them have actually raised €20 M or less, whereas only a tenth have raised over €100 M, mainly through public listings (there are already 20 traded entities worldwide, with 85% of these IPOs happening over the last five years).
Despite the exponential growth in investment in the 2014-2018 period, funding commitment for pharmabiotics almost halved in 2019 compared to the previous year, placing 2019 at the same level of 2015 in terms of both investment and number of IPOs. This trend has also been reflected in the number of microbiome drug startups funded over the last year – 6 new companies versus the average of 22 foundations per year in the period 2014-2018. All this may be due to a natural cycle in high-tech investment. However, the general sense of pessimism around microbiome in Wall Street9 and some recent safety alerts by the FDA and experts on FMT10–12 may also be contributing to this deceleration.
Year 2020 could be apotential turning point for this downward trend, as clinical trials results from major players Seres Therapeutics, Rebiotix (Ferring Pharmaceuticals) and Finch Therapeutics are expected this year. A success story in all or any of these may revive the interest and confidence of the market in this technology. However, given the exceptional situation created by SARS-CoV2, these results may be announced with delay and the financial market may be facing more serious challenges than some lack of clinical endorsement.
Applications: well beyond C. diff
As mentioned above, the microbiome, its composition and/or its activity, have been linked with a vast number of human health conditions1,2. Pharmabiotics companies are aware of all these connections and are engaged in a total of 650 active research programs for tens of different diseases in which the microbiome is believed to play a role.
The massive potential of the use of the human microbiome as a therapeutic strategy gained attention with the publication of the results of a human trial in which fecal transplant (FMT) showed extremely efficacious against recurrent Clostridioides difficile infection13. In fact, not only FMT but also other interventions in which friendly microorganisms are employed to outcompete pathogens have been proven successful over these years. This may be the reason why almost one third of the research programs from pharmabiotics companies are focused on fighting infectious diseases – 30 of them centered on C. difficile alone – which makes infectious disease the main application of microbiome drugs, for now. However, the understanding of the connection between the microbiome and different aspects of human physiology is greater every day, and biotechs are trying to capitalize it.
For instance, deeper understanding is being gained about the impact and contribution of the gut microbiome on the host’s metabolism14. This has enabled that 10% of the research programs be focused on managing metabolic diseases such as diabetes and other components of metabolic syndrome.
Most importantly, it is well known that the microbiome plays a crucial role on the education and correct functioning of the immune system, particularly in the intestine. This is why another 17% of these projects are strategies to treat immune-mediated conditions in the gut such as Inflammatory Bowel Disease (IBD). These applications are indeed capturing the attention of big pharmas like Johnson & Johnson, Takeda, Allergan, Genentech and Abbvie, which have established partnerships with these startups. Nevertheless, microbiome interventions for other diseases of immunity which mainly manifest in different body systems, like multiple sclerosis, rheumatoid arthritis, psoriasis, allergies and asthma are also being investigated.
The relationship between H. pylori and gastric cancer was described decades ago. However, in recent years there has been significant progress in uncovering the relationship between the microbiome and cancer, and we now know that certain microbiome compositions may increase the risk of developing other malignancies, as well as influence the response to cancer therapy15. Oncology is in fact the second main application of microbiome drugs after infectious diseases, with over 100 ongoing projects in cancer. Some companies are focused on finding microbial biomarkers to predict disease risk or monitor disease progression, while others are developing products to actively fight the disease. Some other projects are centered in mitigating the side effects associated with the use of chemotherapy, like diarrhea, or lowering the risk of adverse events of certain therapies like graft-versus-host disease. However, perhaps one of the most promising applications of the microbiome in cancer is recently-found connection between the microbiome and the efficacy of oncology medications. More precisely, there is quite solid evidence about the role of bacteria in the antitumor efficacy of immune checkpoint inhibitors, a relatively new type of medication approved for different kinds of cancer16,17. At least 6 microbiome drug companies are focused on this specific pathway, most of them in partnership with some of the leading big pharmas in oncology such as Merck, Bristol-Myers Squibb, AstraZeneca, Chugai and Astellas.
Lastly, the so-called Gut-Brain Axis is also capturing a great deal of attention in recent times, as more and more evidence is being generated about the cross-talk between the microbiome and the neuroendocrine system and about the influence of the microbiome on mental disease18. Over 40 programs are focused on developing strategies to treat autism, Parkinson’s and Alzheimer’s Disease and depression.
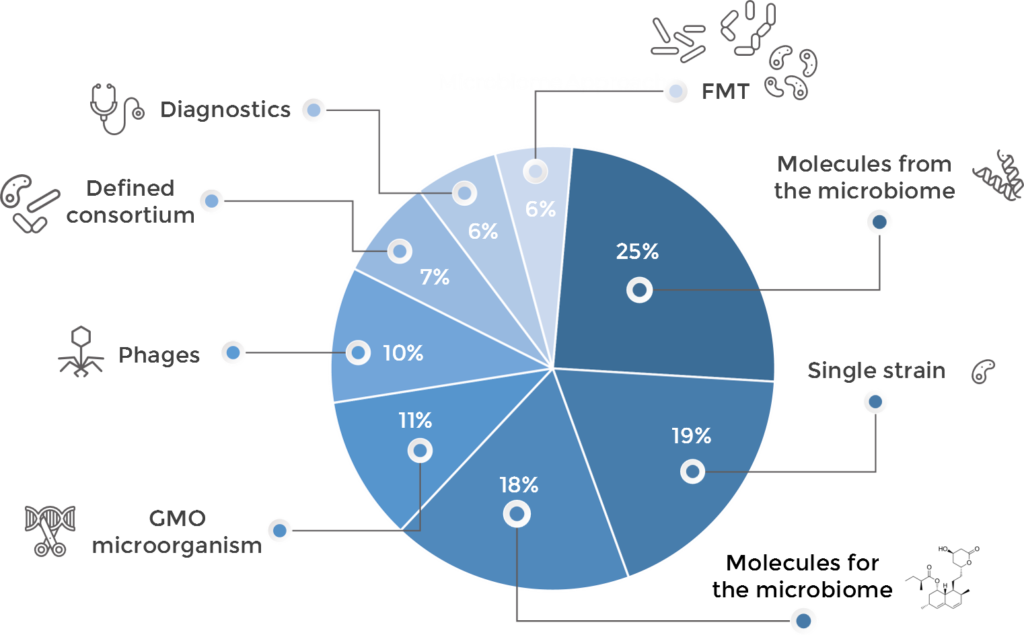
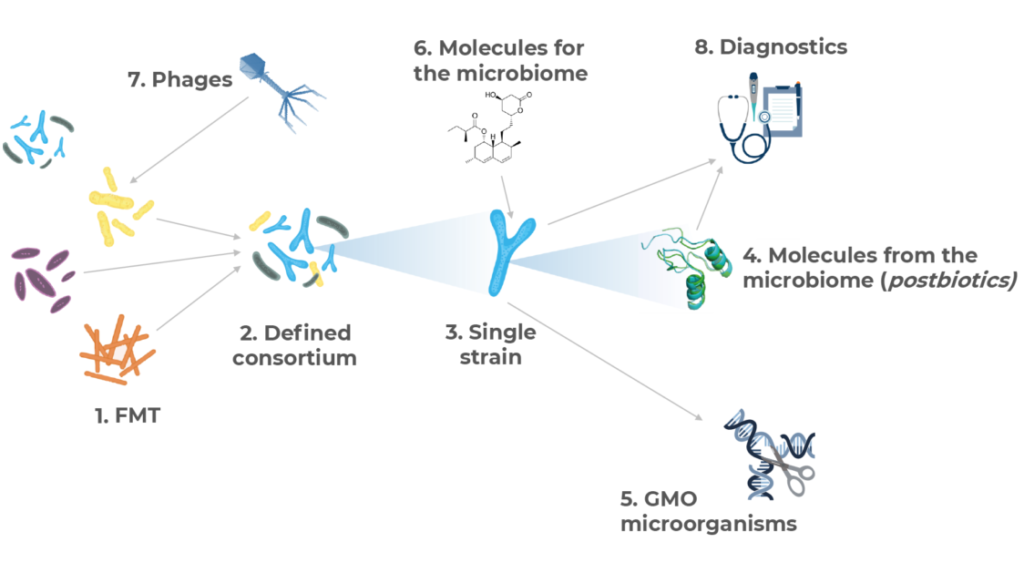
Therapeutic approaches and their connection drug development strategy
The microbiome is a very complex ecosystem with even more complex connections to the human body at the metabolic, immune and neuroendocrine levels. This poses a technical challenge but also an immense opportunity with many different potential approaches to target a plethora of druggable targets. The approach employed to alter the microbiome is critically important as it is a decisive factor in how the new pharmaceutical is regulated. For instance, the FDA ruled that FMT may not be regulated as a drug under certain specific conditions and as long as it is intended to treat C. difficile infection not responsive to standard therapies19. Some of its counterparts in Europe like France, Germany, the UK and Switzerland, however, have stated their opinion that FMT, for any application, must be regulated as a medicinal product. For the remaining approaches detailed below, their regulatory classification varies depending on many different factors such as its chemical or biological structure, composition or characteristics, its origin, its manufacturing process or its mode of action. Given the current lack of specific regulatory frameworks and the lack of harmonization, the precise regulatory allocation must be studied on a case-by-case basis and in a geography-specific manner. For example, what we call below Molecules from the microbiome and Molecules for the microbiome may comprise products that may qualify as small molecules, as biologics or even medical devices, while many products within the Genetically-Modified Organisms (GMO) cluster have been historically treated as gene therapy products by the European Medicines Agency.
Microbiome drug developers are exploring virtually all possible approaches to treat and diagnose disease through the microbiome, using many different strategies which may be clustered as follows:
- FMT: first described in the 4thCentury in China, practiced since the 1950’s and somehow re-discovered recently20,21, this approach involves the transfer of more or less processed fecal microbiome material from a healthy donor to a diseased individual. It was the approach that sparked the interest in curing disease through the microbiome and expanded rapidly in the early days. However, different regulatory, IP and eve safety10,11,22 considerations have made it minoritarian in the industry, with 6% of the companies using it.
- Defined Consortia: this approach is based on treating the patient with a consortium of several microbial species (usually between two and a dozen). Whereas some of these products have emerged from further processing and refinement of FMT, some others have been designed rationally attending at ecological properties, metabolic capacities or other features. Given the technical complexity of these designs, only about 7% of the biotechs are following this strategy to treat disease.
- Single species (strain): inthis strategy, a single type of microorganism is administered to elicit the beneficial effect. It is a popular approach being followed in around 20% of the programs. Most of the programs in this cluster are exploiting the targeted cross-talk between a specific bacterial strain and the immune system to aim at treating inflammatory diseases and cancer. It is also, by far, the main bet from companies developing dermatology drugs.
- Phages: the human phageome, or the collection of viruses targeting bacterial members of the microbiome living in the human body, is being increasingly recognized as an important player in microbiome composition and dynamics. In numbers, there may be at least 109 viruses per gram of human feces, ten times more than that of bacterial cells23. Therefore, using phages to kill bacteria and/or modulate the composition of the microbiome is a strategy that many biotechs are using – around 10% of them do it. The most obvious application of bacteria-killing viruses is fighting infectious disease, and this is indeed the application where most of the phages projects are being researched. However, new applications like oncology and inflammatory diseases are also emerging within this area.
- GMOs: whereas the metabolic capabilities of microorganisms are almost limitless and not yet fully described24, some companies are engineering microbes to turn them into long-term drug delivery systems, or to expand or potentiate their metabolic activity. As a technically and regulatorily challenging approach, only 11% of the analyzed companies use GMOs as therapeutic agents.
- Molecules from the microbiome (also called postbiotics): the members of the microbiome produce tens of thousands of different, chemically diverse substances, most of them yet unknown to man24. Many of them are believed to have significant physiological effect14,25,26 and therefore may have huge pharmacological potential27,28. Arguably the most broadly described molecules produced by microbes are antimicrobials of different chemical nature and enzymes, and these are precisely the main ones that the industry is investigating. Regarding the former, our analysis purposefully excluded developers of antimicrobials, however we identified a number of companies developing bacterial or viral molecules to kill off infectious agents. About the latter, there are a number of enzymes being investigated which aim at breaking down systemically-administered antibiotics in the intestine so that they do not affect the gut microbiome (thus preventing gastrointestinal diseases caused by opportunistic agents) and also others as enzyme replacement therapies for certain metabolic diseases. There are however other microbial derivatives with immune modes of action being developed for inflammatory-mediated maladies and cancer, and also some metabolites being employed to target the gut-brain axis. The great promise of exploring the infinity of potentially active chemicals produced by the microbiome as the new big source of novel therapeutics has been well understood by the industry, and this approach is in fact the most popular one with one in four research programs employing it.
- Molecules for the microbiome: historically, drugs aimed at interfering with microbes had the goal to kill them (e.g. antibiotics). This continues to be the case to a certain extent as many chemicals in this approach target infectious diseases – however in ways different to conventional antimicrobials. Nevertheless, with the deeper understanding of the metabolic activity of the microbiome and its connections with human physiology, there is an increasing trend to apply external chemicals (mostly small molecules) to alter microbial activity and composition29 to manage diseases like immune conditions, IBS and obesity, and to limit the microbial activity over other medications like antineoplastics. This is a growing category, currently employed by almost 20% of the microbiome drug developers, however it is likely that this share expands in the future.
- Diagnostics, companion diagnostics and disease-monitoring technologies: despite the existing cause-vs-consequence debate in the microbiome field, certain microbial signatures and biomarkers have been linked with the risk of developing a disease or with its prognosis. The two main areas where this is being applied by biotechnology companies are colon cancer and IBD, although technologies also exist in diabetes and dermatology.
About Sandwalk BioVentures and the author
Sandwalk Bioventures is a specialty strategy, regulatory and management consulting firm focused on microbiome technologies servicing companies in the food and pharma sectors, as well as financial and strategic investors exploring to enter this field.
Dr. Luis Gosálbez is Managing Partner at Sandwalk BioVentures, Business Development Director at Clasado Biosciences and a contributing author for the Microbiome Times.
—
References
1. Clemente, J. C., Ursell, L. K., Parfrey, L. W. & Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 148, 1258–1270 (2012).
2. Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The Human Microbiota in Health and Disease. Engineering 3, 71–82 (2017).
3. Gosalbez, L. La microbiota humana como estrategia farmacológica en el entorno regulatorio europeo. (2017).
4. Finlay, B. B. Are noncommunicable diseases communicable? Science (80-. ). 367, 250 LP – 251 (2020).
5. O’Hara, A. M. & Shanahan, F. The gut flora as a forgotten organ. EMBO Rep.7, 688–693 (2006).
6. Team, N. I. H. H. M. P. A. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007-2016. Microbiome 7, 31 (2019).
7. Cat, L. A. The decade of the microbiome. Forbes (2019).
8. Pariente, N. & York, A. Milestones in human microbiota research. Nature (2019).
9. Chatsko, M. Will the gut microbiome change medicine? Wall Street isn’t convinced. Motley Fool (2019).
10. US Food and Drug Administration. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse (2019).
11. US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse-events-likely (2020).
12. US Food and Drug Administration. Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Additional Safety Protections Pertaining to SARS-CoV-2 and COVID-19. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-additional-safety-protections.
13. van Nood, E. et al.Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med.368, 407–415 (2013).
14. Visconti, A. et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun.10, 4505 (2019).
15. Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V. & Wargo, J. A. The microbiome, cancer, and cancer therapy. Nat. Med.25, 377–388 (2019).
16. Gopalakrishnan, V. et al.Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science (80-. ).359, 97 LP – 103 (2018).
17. Gong, J. et al.The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin. Transl. Med.8, 9 (2019).
18. Cryan, J. F. et al. The Microbiota-Gut-Brain Axis. Physiol. Rev.99, 1877–2013 (2019).
19. Food and Drug Administration. Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. 1–4 http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM488223.pdf?source=govdelivery&utm_medium=email&utm_source=govdelivery (2016).
20. Kelly, C. P. Fecal Microbiota Transplantation — An Old Therapy Comes of Age. N. Engl. J. Med. 368, 474–475 (2013).
21. Stripling, J. & Rodriguez, M. Current evidence in delivery and therapeutic uses of fecal microbiota transplantation in human diseases-Clostridium difficile disease and beyond. Am. J. Med. Sci. 356, 424–432 (2018).
22. Ianiro, G. et al.Role of yeasts in healthy and impaired gut microbiota: the gut mycome. Curr. Pharm. Des.20, 4565–4569 (2014).
23. Wylie, K. M., Weinstock, G. M. & Storch, G. A. Emerging view of the human virome. Transl. Res.160, 283–290 (2012).
24. Sberro, H. et al. Large-Scale Analyses of Human Microbiomes Reveal Thousands of Small, Novel Genes. Cell 178, 1245-1259.e14 (2019).
25. Wikoff, W. R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. (2009) doi:10.1073/pnas.0812874106.
26. Wilmanski, T. et al. Blood metabolome predicts gut microbiome α-diversity in humans. Nat. Biotechnol. 37, 1217–1228 (2019).
27. Turroni, S., Brigidi, P., Cavalli, A. & Candela, M. Microbiota–Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 61, 47–61 (2018).
28. Wang, L., Ravichandran, V., Yin, Y., Yin, J. & Zhang, Y. Natural Products from Mammalian Gut Microbiota. Trends Biotechnol. 37, 492–504 (2019).
29. Cully, M. Microbiome therapeutics go small molecule. Nat. Rev. Drug Discov.18, 569–572 (2019).

Luis Gosálbez
Sandwalk BioVentures is a specialty strategy, innovation, regulatory and management consulting firm focused on microbiome technologies servicing companies in the food and pharma sectors, as well as financial and strategic investors exploring to enter this field. The company has created the Microbiome Drug Database™, an online repository containing the most extensive and thorough analysis of biotechnology companies developing pharmaceuticals from or through the microbiome.
