While“healthy” microbiome remains to be defined, microbiome disruption is associated with numerous diseases, offering new therapeutic opportunities if causalities are demonstrated. Europe is at the forefront of the understanding of the Microbiome and is well placed to play a major role in the future, be it for Nutrition or Pharmaceutical applications.
Among the ~2,100 microbiome clinical trials listed on the clinicaltrials.gov global database from 2000 to 2017, 35% of the studies are conducted in Europe, followed by 33% in the USA. China is third with only 5% of the overall number of studies. Nonetheless, the number of Chinese clinical studies is thought to be underestimated. Indeed, after merging the previous database with the Chinese Clinical Trials Registry, the number of Chinese microbiome clinical studies is twice higher representing about 10% of the overall microbiome studies.
Europe was responsible for the initiative of the first trial mentioning microbiome in 2000, while the USA tackled the subject in 2006 and China in 2009. Moreover, Europe represents 32% of the publications on the microbiome listed on PubMed from 2006 to 2017, versus 23% for the USA and 9% for China.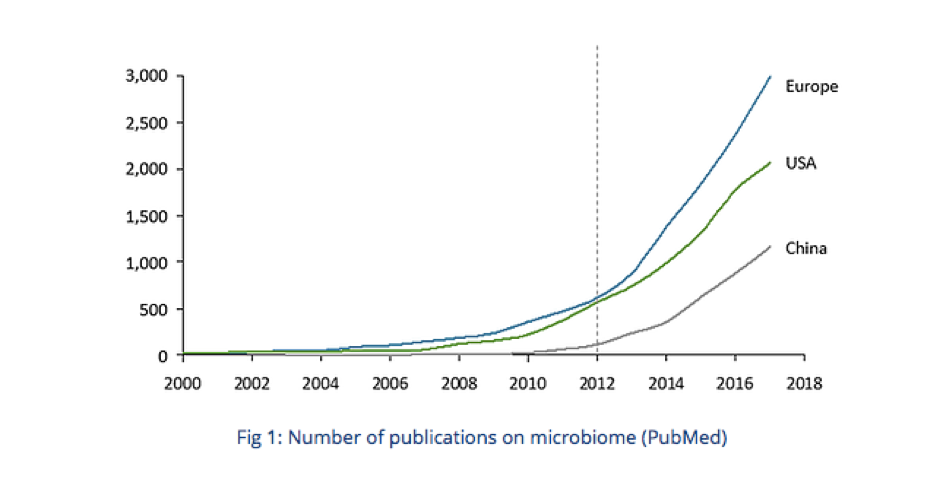
Initially, microbiome therapeutic areas of interest have been gastrointestinal diseases (irritable bowel syndrome, inflammatory bowel disease, and Crohn’s disease) and metabolic diseases (obesity and diabetes). They respectively represent 20% and 15% of the overall number of microbiome trials performed in Europe and the USA (2000-2017 period).
In recent years, the potential applications for microbiome have been extended to other therapeutic areas. Based on the number of clinical trials (Clinicaltrials.gov), the therapeutic areas that progress the fastest are central nervous system diseases, infectious diseases and oncology. The number of clinical trials in oncology, including a microbiome analysis, is growing at a fast pace in Europe with +41% p.a. since 2011, but it is still behind the USA in volume. Indeed, microbiome trials in oncology already represent 7% of the overall number of trials conducted since 2000 in the USA while it represents just 2% in Europe.
The first microbiome-based therapeutic treatments are already emerging, such as the fecal microbiota transplantation (FMT). Most of the interventions consist of introducing the microbiota from a healthy donor into a patient. The FMT is successfully used to treat Clostridium difficile infections (CDI) but it could also be applied to counteract gut dysbiosis following heavy treatments like chemotherapy. For instance, Maat Pharma, OpenBiome, or Rebiotix are biotechs developing FMT solutions. It requires the access to a biobank with characterized feces from healthy donors. However, the legislation around FMT is still to be defined. In the USA, it is classified as a biologic product and a drug only for CDI. In Canada, it is registered as a new biologic drug and its use is restricted to clinical trial. There is no regulation at the European level, up to date the countries are setting their own rules. In France, it is considered to be a drug and aside from exceptional circumstances, FMT should only be administrated under a clinical trial. However, in the UK, the National Institute for Health and Care Excellence (NICE) allows the use of FMT in the NHS (National Health Service) for patients with recurrent CDI who are not responding to traditional therapies. With more clinical trials, regulations about FMT therapeutic solutions should be revisited in the future.
Besides the FMT, the Pharma industry is also interested in other microbiome therapeutic solutions, such as the ‘phagotherapy’, the antimicrobial peptides, and therapeutic adjuvant.
The interest of microbiome is not limited to the scope of the Pharma industry. The Food industry is actively contributing to the research on the microbiome. Indeed, the Food industry holds a significant part in the emerging of microbiome clinical trials. Among the 15 clinical trials mentioning “microbiome” in Europe in 2008, 14 were related to the Food industry. Moreover, 57% of European overall studies related to microbiome from 2000-17 come from the Food industry. For the same period, in the USA the cumulated number of food microbiome trials represents 43% of the total. In both the USA and Europe, microbiome clinical trials related to the Food industry accounts for about 50% of the studies in 2017.
The interest of the Food industry in the microbiome relies mostly on the development of probiotics and prebiotics.Clinicaltrials investigate the role of different diets like the Western diet, Mediterranean diets, vegan diets, or gluten free diets on the microbiome. However, since the reinforcement of the European Food Safety Authority (EFSA) regulation on health claims in 2012, the number of food clinical studies is stagnating. Yet some Food companies such as PiLeJe are developing their products as a drug and therefore follow the same path as Pharma companies with clinical studies to prove the efficiency and safety of their products.
Besides human health, another microbiome research area is emerging in the food industry, which is the food quality in the production plant. How to control the microbial ecosystems in food production to optimize sensory flavor of the products better? How to control spoilage bacteria? The Researchers in the food industry need to better understand the relationship between shelf-life and microbial behavior. Advanced technologies such as the uses of phages or antimicrobial peptides (AMPs) are studied to target specific bacteria to prevent undesirable bacterial colonization (biopreservation).
Pharma and food industry interest inMicrobiome and human health is booming. Europe is at the forefront of human microbiome research, as it promotes the emergence of new biotech companies and developing microbiome-based products. However, human microbiome-related research activitiesand clinical trials take place in large volumes also in the USA and the restof the world. The interest and engagement in the study and further understandingof human microbiome (and its clinical applications) is constantly increasing, both within the pharma andthe food industry. In the future, pharma and food industries might cross-fertilize their microbiome discoveries and work together for standardization methodologies for a better understanding of microbiome ecosystems and functionalities.
Find more content on microbiome science market analysis on biofortis’s blog
References:
- Human Microbiome Project website
- Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B. (2016) Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion. 56:1138-47. doi: 10.1111/trf.13477.
- Aragón IM, Herrera-Imbroda B, Queipo-Ortuño MI, Castillo E, Del Moral JS, Gómez-Millán J, Yucel G, Lara MF. (2016) The Urinary Tract Microbiome in Health and Disease. Eur Urol Focus. pii: S2405-4569(16)30159-6. doi: 10.1016/j.euf.2016.11.001.
- Nguyen LD, Viscogliosi E, Delhaes L (2015) The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol. 13;6:89. doi: 10.3389/fmicb.2015.00089.
- Kumar A, Chordia N (2017) Role of Microbes in Human Health. Appli Microbiol Open Access 3:131. doi:10.4172/2471-9315.1000131
Sources:
- Clinicaltrials.gov as of January 2018
- Chinese Clinical Trial Registry as of January 2018
- PubMed as of January 2018



