
Abstract
Microbiome drug development has captured significant interest over the last decade. However, despite the recent clinical and regulatory success stories, this area is lately regarded as a high-risk one by investors given the current economic situation. Therefore, evidence-based de-risking strategies for pipelines and portfolios are necessary.
Here we present the first-ever analysis of microbiome drug development success rates based on the historical pipeline. The analysis provides preliminary insights on what therapeutic applications and drug modalities seem to be more promising, while highlighting the bottlenecks in microbiome drug development.
Introduction

In 2013 interest in the development of drugs based on microbiome strategies rose exponentially with the publication of the results of a clinical trial in which Fecal Microbiota Transplantation (FMT) proved significantly more efficacious than the standard-of-care, vancomycin, in the treatment of recurrent Clostridioides difficile infection [1]. Although the role of the microbiome in health and disease was already inferred, and FMT had shown promising results in C. difficile infections long before [2-6], it has been these last 10 years when significant attention has been paid to microbiome drugs. Capital investment has accompanied interest. In fact, funding from both financial and strategic investors in microbiome drug development companies has continuously grown year after year over the last decade, peaking up in the period 2021-2022.
The first market authorizations for microbiome drugs occurred in 2022. BiomeBank received the approval for its FMT product Biomictra™ targeting recurrent C. difficile infection by Australia’s Therapeutic Goods Administration on November 9th 2022 [7]. The first authorization in the USA was for Ferring’s-Rebiotix’s Rebyota™ (known as RBX2660 as an investigational drug), also for recurrent C. difficile infections [8]. Italy’s Proge Farm received a market authorization in Switzerland for its single strain L. plantarum P17630 (Softigyn®) as a topical treatment for vaginitis and prevention of recurrent vaginal mycosis. Between 2022 and early 2023, a topical product also indicated for vaginal infections containing L. gasseri and L. rhamnosus was authorized as a drug in Germany and Spain as a reclassification from its former medical device status in Europe [9]. Last month, Seres Therapeutics received the US FDA’s approval for VOWST™ (known as SER-109 as an investigational drug) for recurrent C. difficile infections, thus becoming the first orally-administered microbiota-based authorized drug [10].
At the start of the COVID pandemic, investment into biotech research boomed. However, over the past few months we are seeing investment return to pre-pandemic levels. After two years of an investment-rich climate, Venture Capital investment in life sciences, biotech and therapeutics dropped by 20-30% in 2022 [11] and remains plateaued into 2023. In the microbiome drug development sector, this has led to up to 10% of the formerly active companies leaving the space over the last year. Whilst some have been forced to terminate operations due to their inability to raise follow-on financing, others are now pivoting to other areas of drug development such as monoclonal antibodies, oncolytic viruses and mRNA, while others have compensated by adding or changing to a fee-for-service business model within microbiome sciences.
Given this precision financing environment, it is important to provide biotechs and investors with data to enable informed decisions to de-risk their pipelines and portfolios [12].
We performed an analysis in which we highlight the bottlenecks in microbiome drug development, compare microbiome drugs with the broader biotherapeutics sector and identify the therapeutic applications and approaches in which microbiome-based strategies we predict to have higher chances of succeeding.
Analysis of the historical pipeline and success rates calculation
Since 2016, Sandwalk BioVentures has systematically tracked all microbiome drug companies, and their respective development programs [13]. To date, this totals 2,020 programs, 65% of which are still active today or have reached market.
We analyzed the data from all active and discontinued programs from 365 companies – of which 15% are currently inactive or no longer active in microbiome drug development. This is, to our knowledge, the first time real industry data has been applied for this analysis, and the first time a head-to-head comparison is made between microbiome drug development and the broader pharmaceutical industry. It should be highlighted that given the infancy of the microbiome industry, these results must be interpreted with extreme caution, and that certain metrics cannot yet be calculated due to the absence of data critical mass to do so.
Development success or phase transition rates are one of the main metrics employed by the pharmaceutical industry to measure R&D outcomes. Phase transitions occur when a drug candidate advances into the next phase of development. Programs that fail to transition are discontinued. We calculated phase transition success rates (how likely a given development program is to transition from each individual phase to the next one) by comparing the number of programs progressing to the next phase vs. the total number of programs (including those progressing and being discontinued at each development stage). Conversely, attrition rate shows the likelihood of programs failing to transition.
Given the absence of data, it is not yet possible to calculate these metrics for Phase 3, and the regulatory approval stages for most industry segments, so information is mostly shown for Phases 1, and 2. We benchmarked these metrics against those calculated by other publications [14-20] for the broader (i.e. non-microbiome related) pharmaceutical development pipeline.
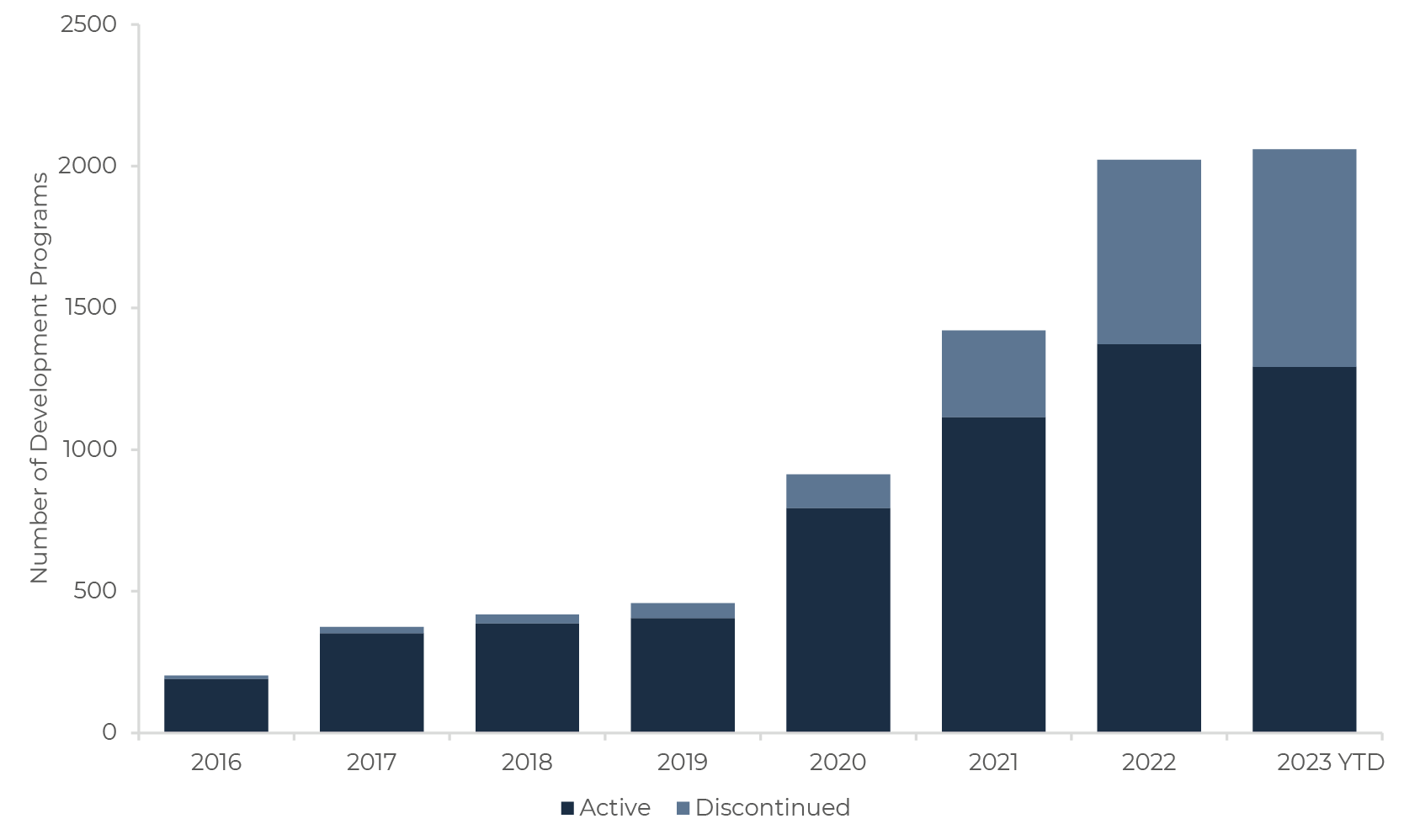
Analysis highlights and limitations
Microbiome-based drugs are a highly heterogeneous therapeutic group (covering a wide range of products which massively diverge in their biochemical complexity, from FMT to molecular approaches), with diverse application routes (oral, rectal, topical, and injected), and targeting many different indications (from IBS to solid tumors). To reduce confounding factors and obtain more meaningful results, we analyzed the overall historical pipeline of microbiome drugs but emphasized comparisons between different drug modalities, drug’s different applications. We also compared the success rates of microbiome with their closest counterparts in the general therapeutic development sector.
It is noteworthy that most of the available information about microbiome drug development is related to earlier-stage programs (i.e. Discovery, Preclinical and somewhat Phase 1, as few programs have had the physical time to reach Phases 2 and 3, see Figure 2). In the earliest stages of drug development, data transparency is often limited in the microbiome and otherwise pharmaceutical industry, so success rates may be overestimated. This seems to be the case given the extraordinarily high observed success rates for the Discovery and Preclinical stages, much higher than the ones for the broader drug development sector [20].
For interpretation of results, we worked with the assumption that success rates at each stage were informative of their respective main primary goals (i.e. safety, efficacy, dose-finding, etc). Nonetheless, reality is more complex and on many occasions explanations of the reasons for success or failure are lacking.
Therefore, this analysis must be interpreted cautiously. It is, however, the best dataset we have for now. The trends identified are detailed below.
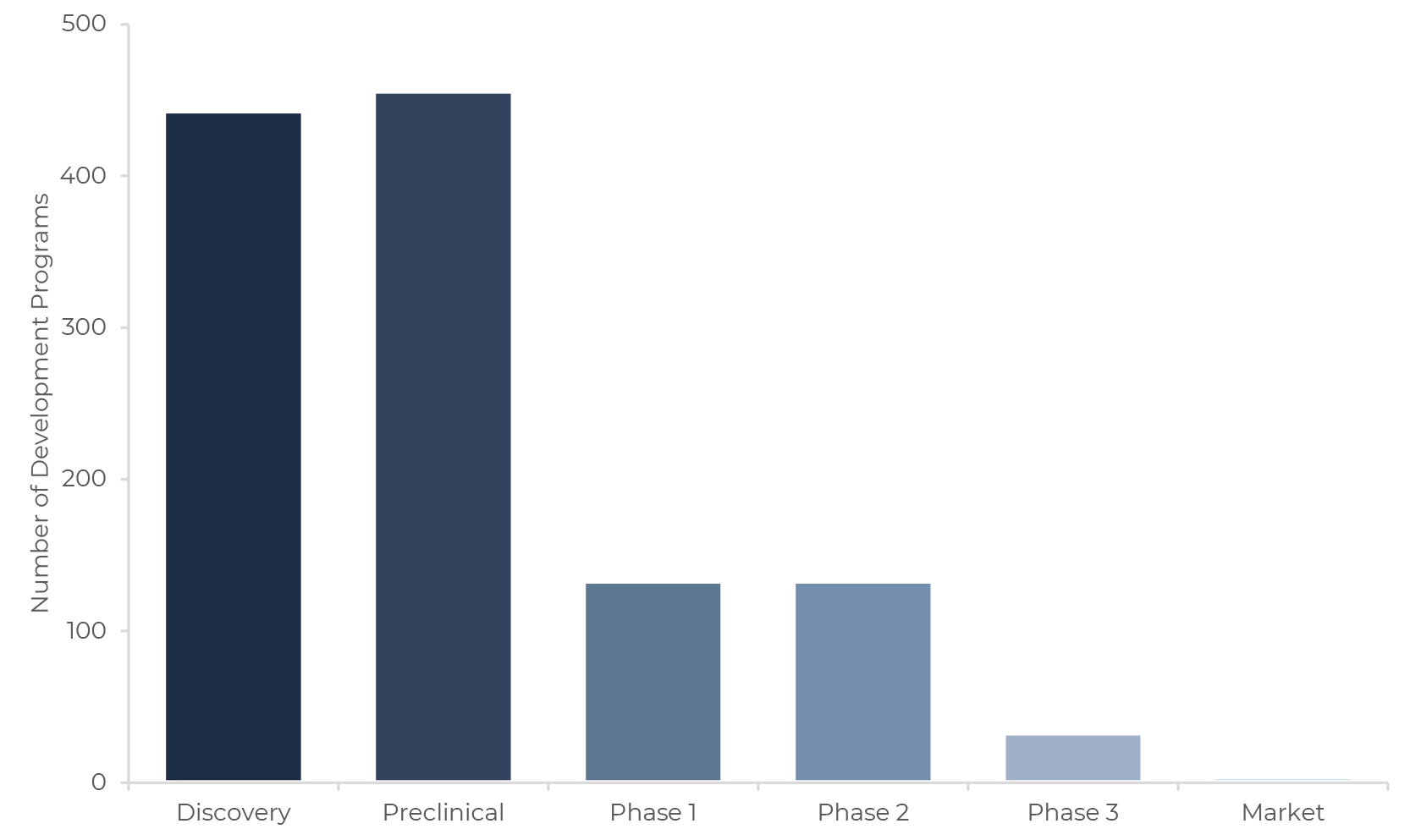
1. Microbiome-based drugs tend to be safe
There is a general perception that microbiome-based therapies are safe, mainly because some are derived from functional food ingredients. Our data suggests this is the case, as microbiome drugs seem to outperform other drugs in Phase 1 success rate – over 80% of all microbiome-based drugs that enter this stage successfully complete it and transition to Phase 2. In fact, a significant share of microbiome drug development programs are waived from conducting Phase 1 and enter Phase 2 directly. Exceptionally high success rates in Preclinical also point in the same direction. However, as stated above, this result is likely an artefact emerging from the limited public data for this development stage.
2. Differences in success rates do indeed exist between therapeutic applications
Perhaps unsurprisingly given the evidence of the importance of the gut microbiome in intestinal physiology, we found that the therapeutic application where microbiome drugs seem to have the highest chances to succeed from Discovery to Phase 3 is Gastrointestinal diseases. They outperform all classes of microbiome drugs for all applications in all stages, and match gastrointestinal drug candidates acting through microbiome-independent mechanisms in Phase 2 [14]. These microbiome drugs are indicated mainly for Irritable Bowel Syndrome (IBS), Celiac Disease, Small Intestinal Bacterial Overgrowth (SIBO) and Gastroesophageal Reflux Disease (GERD), amongst many others. This reinforces the notion that studying the gut microbiome is important to understand and tackle gastrointestinal disorders. Importantly, microbiome-based gastrointestinal drugs perform significantly better than microbiome-independent drugs in Phase 1 – their success rate is roughly double other drugs at this stage. This is promising as Gastroenterology holds a top-three position in Phase 1 attrition rate in drug development [13].
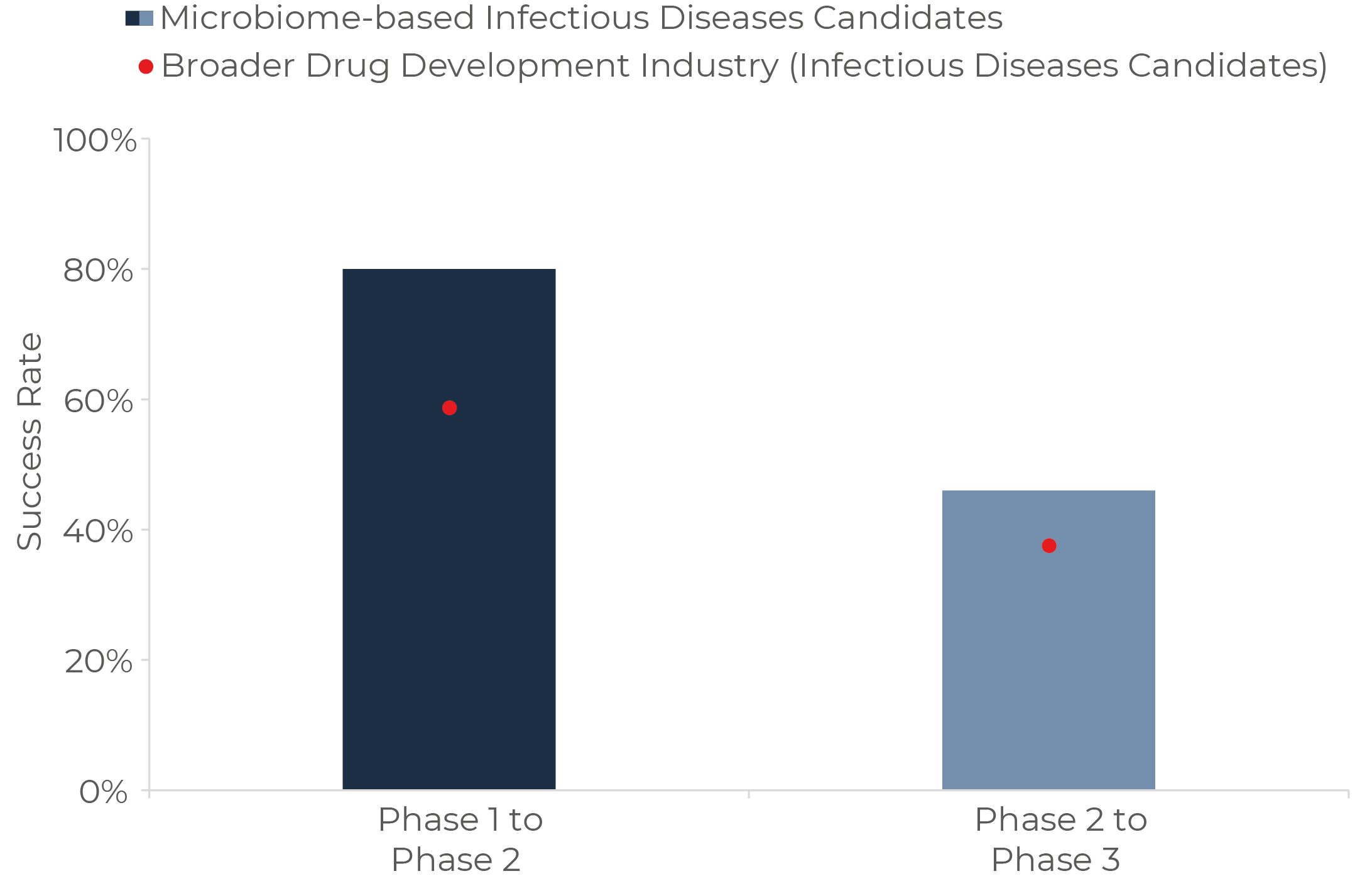
For Phase 2 success rates, Infectious diseases is the application cluster in which microbiome-based therapeutics stand out. These include FMT and donor-derived therapies for C. difficile infection, bacteriophage treatments for antibiotic-resistant bacteria, amongst others. As depicted in Figure 3 below, it appears fighting infections with microbiome-based strategies has a 20% higher probability of success than microbiome-independent modalities (e.g. broad-spectrum antibiotics, antivirals and others).
Regarding the applications that have captured most interest and investment over the last years, Autoimmunity and Oncology, results are mixed with exceptionally high transition rates in Phase 1 and significantly more modest outcomes in Phase 2.
3. Differences in success rates do also indeed exist between microbiome drug modalities
We ran the same analysis comparing the success rates of different approaches used to target or manipulate the microbiome. There are three findings that stand out.
The first is the relatively low success rate of Bacteriophages in Phase 1. This is surprising since phages are regarded as a safe approach to treat infections when used orally, topically or intravenously [21-26], and several examples of successful use have been published over the last 18 months [27-29]. However, Phase 2 success for Phages is aligned with the rate for the overall pharmaceutical market.
A possible reason for this is that most of the published success cases are based on the, often compassionate, use of phages in individual patients or small populations, and safety concerns, perhaps related to the presence of bacterial toxins from manufacturing in the product [24] or immunogenicity [30], may emerge only in larger trials. Challenges in defining dosage levels may also be behind this high attrition rate, as this is a complex subject in phage therapy. Initiatives to explore the safety and the quality of phages as drug active substances have been recently established both in Europe and in the US [31-33].
The second insight is the exceptionally low success rate of “Molecules from the microbiome” / drugs from bugs in Phase 2. This approach shows sky-high transition rates from Discovery throughout Phase 1 (Figure 4), followed by an extremely low Phase 2 success rate (around 70-75% lower than the one for any other class of drug). On the other hand, “Molecules for the microbiome” or drugs for bugs (defined as non-microbiome derived molecules employed to change the microbiome in a pharmaceutical way, excluding all classical and broad-spectrum antibiotics, as well as other drugs which may have an impact on the microbiome but that do not have it as their main target) show very good success rates across the board when compared with microbiome-independent drug candidates.
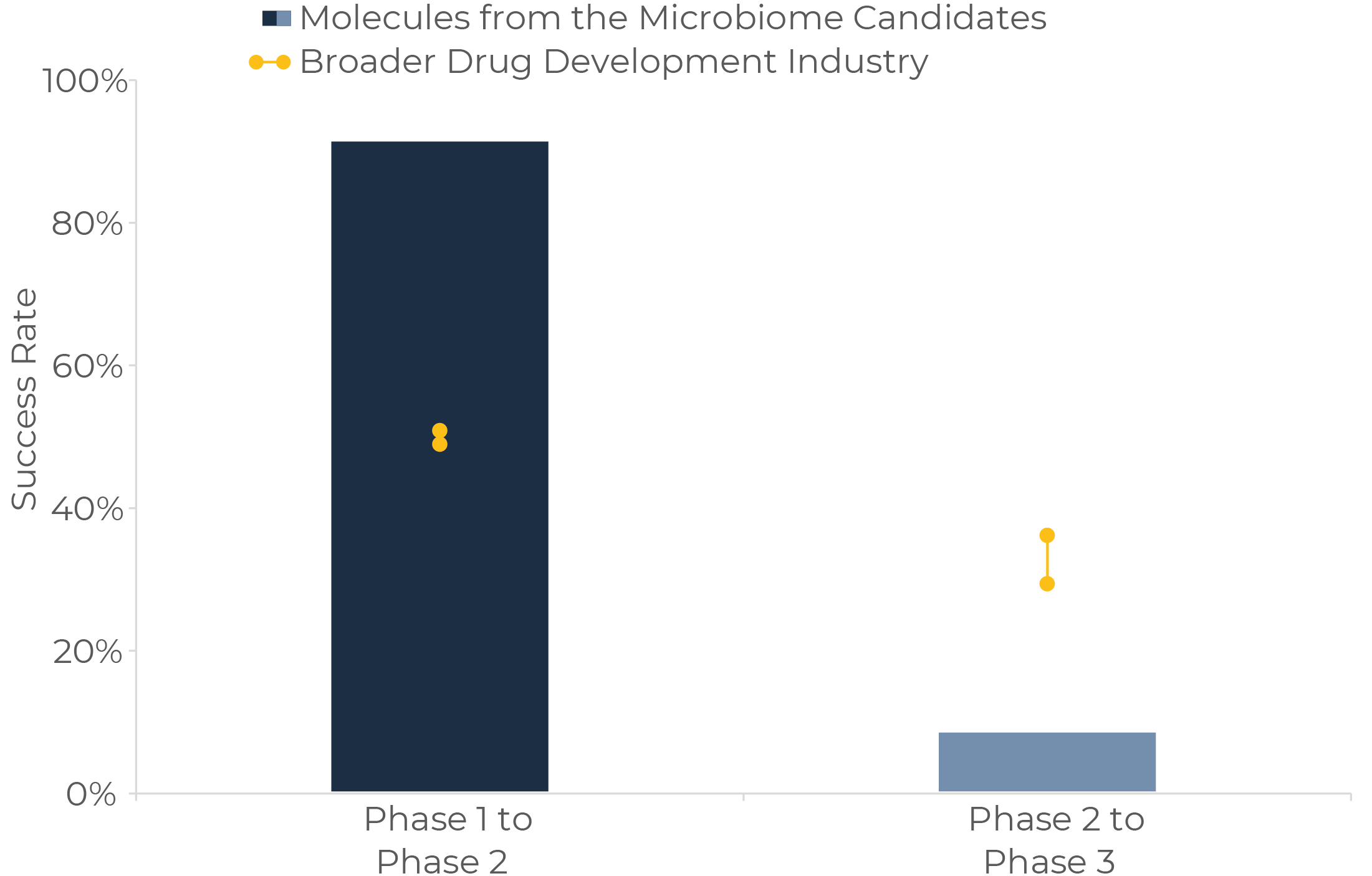
Looking at the specifics, a potential explanation for these phenomena may be that many of the Molecules from discontinued programs were based on substances with poor drug-like properties, such as orally-administered microbial enzymes for different therapeutic applications. On the contrary, Molecules for tend to be derived from better-known drug chemical backbones, which makes it easier to predict safety, efficacy and sometimes mechanisms of action. These learnings may be particularly relevant for the industry as the “Molecules from the microbiome” approach is the one receiving most financing over the last months. Other companies capturing the most attention include those focused on the identification of biosynthetic gene clusters for small molecule mining, or on the discovery of novel gene-editing systems in microbiomes to apply them in gene therapy. These novel approaches seem indeed very promising, although they will surely face their own specific challenges.
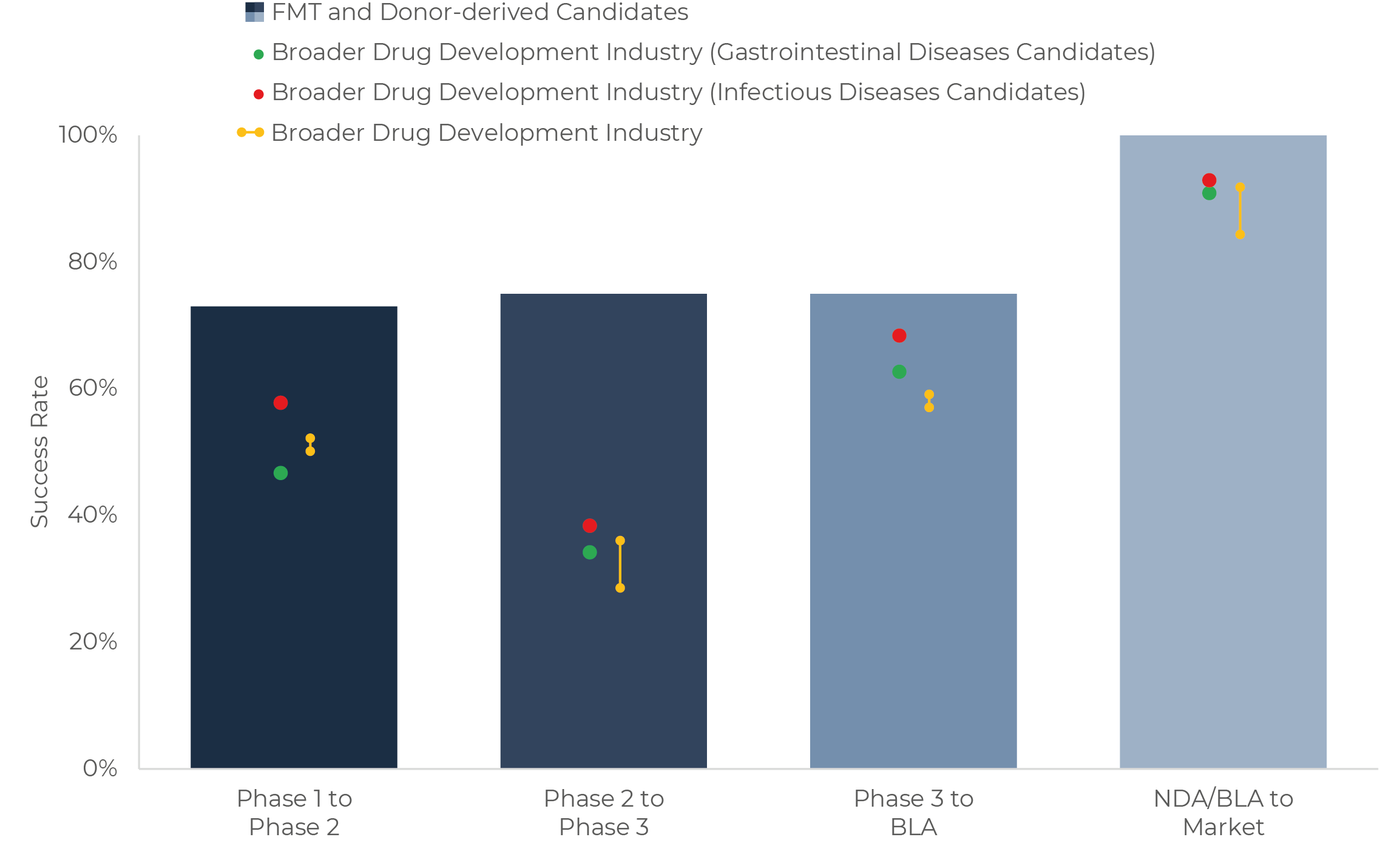
The third highlight is the good performance of FMT and donor-derived products in all stages. Although data is still scarce, the relative maturity of this modality in the microbiome therapeutics area and the recent regulatory approvals for this class allows for preliminary estimate success rates all the way up to Market.
In Figure 5 we represent the performance of this modality against candidates for infectious and gastrointestinal diseases in the overall pharmaceutical sector, which are the closest comparable categories. We also benchmark them against the overall drug development metrics for broader context.
An observed BLA-to-Market success rate of 100% so far looks relieving for the microbiome therapeutics industry, as the lack of specific regulatory pathways for these drugs has been a historical concern for the sector and it’s investors [34]. This success rate shows microbiome drugs can reach the market when they prove safe and efficacious. The path has been long and difficult however. Resulting from decade-long efforts from companies and organizations with regulators. Challenges have been particularly large for donor-derived products, as the COVID pandemic led to increased concerns regarding transmission of infectious diseases [35]. How regulators in Europe react to FMT and donor-derived products also remains to be seen, although progress is being made [36].
Furthermore, success rates from Phase 1 to Phase 3 look quite exceptional for FMT and donor-derived therapies when compared to their closest peers in non-microbiome drug development. However, few programs have yet reached Phase 2 or 3, or are ongoing, so optimism should remain moderate.
It should also be mentioned that all FMT and donor-derived products authorized to date are designed for a single indication – C. difficile infection. As discussed in the beginning, these are the modality and the application which started the rush for microbiome drugs, and the mechanism of action of these drugs, mostly based on competitive exclusion of the pathogen, is relatively simple. Multiple companies are currently exploring the capacity of FMT and donor-derived products in a wide range of applications beyond C. difficile infection. For example, a Phase 3 trial will soon start on Graft-versus-Host Disease [37] and multiple Phase 2 and Phase 1 studies are ongoing for conditions such as IBD, liver diseases, depression and as an adjuvant therapy to medications for different malignancies like lung cancer and melanoma. In all these, mechanisms of action are very different from the ones involved in FMT for C. difficile infection, and their efficacy remains to be confirmed. Nevertheless, the existing literature calls for optimism.
Conclusions
The microbiome drug industry is still in its infancy, but our preliminary analysis highlights microbiome-based drugs tend to be safe and show promise particularly in gastroenterology and infectious diseases. It also underlines that, whereas FMT and donor-derived approaches seem clinically successful, bottlenecks still exist in more novel and promising drug modalities like bacteriophages and new chemistry emerging from microbiomes (drugs from bugs).
It should be considered the promising areas identified, namely FMT and donor-derived approaches, as well as gastrointestinal and infectious diseases applications, are the ones in which microbiome science is more mature and advanced. Therefore, emerging areas of microbiome research like the microbiome-immune system relationship, microbiome-immune-therapy interactions, and the gut-brain axis may simply need more time to develop. Time will tell.
References
1) van Nood, E. et al. “Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile” N Engl J Med 2013; 368:407-415 January 2013, doi: 1056/NEJMoa1205037
2) van Nood, E. et al. “Struggling with recurrent Clostridium difficile infections: is donor faeces the solution?” Euro 2009;14(34):pii=19316. doi.org/10.2807/ese.14.34.19316-en
3) Aas, J. et al. “Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube” Clin Infect Dis. 2003 Mar 1;36(5):580-5. doi: 1086/367657. Epub 2003 Feb 14. PMID: 12594638
4) Rohlke, F. et al. “Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology” J Clin 2010 Sep;44(8):567-70. doi: 10.1097/MCG.0b013e3181dadb10. PMID: 20485184
5) Garborg, K. et al. “Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated ” Scand J Infect Dis. 2010 Dec;42(11-12):857-61. doi: 10.3109/00365548.2010.499541. Epub 2010 Jul 22. PMID: 20662620
6) Gough, et al. “Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection” Clin Infect Dis. 2011 Nov;53(10):994-1002. doi: 10.1093/cid/cir632. PMID: 22002980.
7) BiomeBank Announces World-First Regulatory Approval for Donor-Derived Microbiome Drug. Business November 9, 2022. https://www.businesswire.com/news/home/20221108006340/en/BiomeBank-Announces-World-First-Regulatory-Approval-for-Donor-Derived-Microbiome-Drug
8) FDA Approves First Fecal Microbiota FDA. November 30, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product
9) Vaginal probiotics quietly making progress in drug reclassification in the Sandwalk Bioventures. May, 22, 2023. https://www.sandwalkbio.com/post/vaginal-probiotics-quietly-making-progress-in-drug-reclassification-in-the-eu
10) Seres Therapeutics and Nestlé Health Science Announce FDA Clearance of Investigational New Drug Application for SER-109 in difficile Infection. Business Wire. April 26, 2023. https://ir.serestherapeutics.com/news-releases/news-release-details/seres-therapeutics-and-nestle-health-science-announce-fda
11) Biotech Companies Hunt for Cash as VC Funding Dries Up. Bloomberg. January 18, 2023. https://www.bloomberg.com/news/articles/2023-01-18/biotech-companies-hunt-for-cash-as-vc-funding-dries-up#xj4y7vzkg
12) How gut microbes impact the brain and Nature. April 27, 2023. https://www.nature.com/articles/d41587-023-00001-z?utm_source=linkedin&utm_medium=social&utm_content=organic&utm_campaign=CONR_JRNLS_AWA1_GL_SCON_SMEDA_NATUREPORTFOLIO
13) The Microbiome Times, Microbiome Drug Database https://www.microbiometimes.com/drug-database-2/mdd/
14) Biotechnology Innovation Organization “Clinical Development Success Rates and Contributing Factors 2011–2020”, February 2021, https://go.bio.org/rs/490-EHZ-999/images/ClinicalDevelopmentSuccessRates2011_2020.pdf
15) Sun, et al. “Why 90% of clinical drug development fails and how to improve it?” Acta Pharmaceutica Sinica B vol. 12, 7 3049-3062. July 2022, doi: 10.1016/j.apsb.2022.02.002
16) Kimmitt R, Vieira M. “Research Synthesis: Time and Success Rates of Pharmaceutical R&D”, Knowledge Network on Innovation and Access to Medicines, March 2021, updated July 2021, https://www.knowledgeportalia.org/_files/ugd/356854_9dd6f18e2b114015b253736b1c666cfb.pdf
17) Paul, S.M. et al. “How to improve R&D productivity: The pharmaceutical industry’s grand challenge” 2010 Nature Reviews Drug 9(3), 203-214. https://doi.org/10.1038/nrd3078
18) Mestre-Ferrandiz, J. et al. “The R&D Cost of a New Medicine” 2012 Monograph 000135, Office of Health
19) Loewa, A. et al. “Human disease models in drug development” 2023 Nat Rev Bioeng. https://doi.org/10.1038/s44222-023-00063-3
20) Van Norman, G.A. “Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials” JACC Basic Transl Sci. 2020 Apr;5(4):387-397. doi: 10.1016/j.jacbts.2020.03.010. Epub 2020 Apr 27. PMID: 32363250; PMCID: PMC7185927.
21) Mehmood Khan, F. et al. “The applications of animal models in phage therapy: An update, Human Vaccines & Immunotherapeutics” 2023 19:1, DOI: 1080/21645515.2023.2175519
22) Biswa, B. et al. “Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium” Infect 2002 Jan;70(1):204-10. doi: 10.1128/IAI.70.1.204-210.2002. Erratum in: Infect Immun 2002 Mar;70(3):1664. PMID: 11748184; PMCID: PMC127648
23) Aslam, S. et al. “Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States” Open Forum Infect Dis. 2020 Aug 27;7(9):ofaa389. doi: 10.1093/ofid/ofaa389. PMID: 33005701; PMCID: PMC7519779
24) Merril, R. et al. “Long-circulating bacteriophage as antibacterial agents” Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3188-92. doi: 10.1073/pnas.93.8.3188. PMID: 8622911; PMCID: PMC39580
25) Sarker, S.A. et al. “Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh” 2016 Jan 5;4:124-37. doi: 10.1016/j.ebiom.2015.12.023. PMID: 26981577; PMCID: PMC4776075
26) Microbiome Editing for Infectious Disease and More: An Overview of Bacteriophage Drug Microbiome Times. December 11, 2021 https://www.microbiometimes.com/microbiome-editing-for-infectious-disease-and-more-an-overview-of-bacteriophage-drug-development/
27) Van Nieuwenhuyse, et al. “Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler” Nat Commun 13, 5725 (2022). https://doi.org/10.1038/s41467-022-33294-w
28) Dedrik, M. et al. “Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease”, Clinical Infectious Diseases, Volume 76, Issue 1, 1 January 2023, Pages 103–112, https://doi.org/10.1093/cid/ciac453
29) Chan, K. et al. “Phage treatment of an aortic graft infected with Pseudomonas aeruginosa” Evol Med Public Health. 2018 Mar 8;2018(1):60-66. doi: 10.1093/emph/eoy005. PMID: 29588855; PMCID: PMC5842392
30) Champagne-Jorgensen, K. et al. “Immunogenicity of bacteriophages. In Trends in Microbiology” 2023 Elsevier BV. https://doi.org/10.1016/j.tim.2023.04.008
31) Public consultation on new general chapter on phage therapy active substances and medicinal products for human and veterinary use in Pharmeuropa 2. European Directorate for the Quality of Medicines & HealthCare. April 6, 2023, https://www.edqm.eu/en/-/public-consultation-on-new-general-chapter-on-phage-therapy-active-substances-and-medicinal-products-for-human-and-veterinary-use-in-pharmeuropa-35.2
32) NIH Awards Grants to Support Bacteriophage Therapy National Institute of Allergy and Infectious Diseases. October 19, 2021. March, 11, 2021 https://www.niaid.nih.gov/news-events/nih-awards-grants-support-bacteriophage-therapy-research
33) NIH-supported clinical trial of phage therapy for cystic fibrosis National Institutes of Health. October 4, 2022. https://www.nih.gov/news-events/news-releases/nih-supported-clinical-trial-phage-therapy-cystic-fibrosis-begins
34) Cordaillat-Simmons, et al. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med 52, 1397–1406 (2020). https://doi.org/10.1038/s12276-020-0437-6
35) Fourth safety alert of FDA on FMT in less than a Sandwalk BioVentures. April 10, 2020. https://www.sandwalkbio.com/post/fourth-safety-alert-of-fda-on-fmt-in-less-than-a-month
36) Fecal Microbiota Transplantation (FMT) Global Industry and Regulatory Sandwalk BioVentures. June, 27, 2022. https://www.sandwalkbio.com/post/fecal-microbiota-transplantation-fmt-global-industry-and-regulatory-overview
37) MAAT Pharma Announces S. FDA Lifts Clinical Hold on Phase 3 Investigational New Drug Application for MAAT013 in Patients with Acute Graft-versus-Host Disease. April 24, 2023. https://www.maatpharma.com/april-24-2023-maat-pharma-announces-u-s-fda-lifts-clinical-hold-on-phase-3-investigational-new-drug-application-for-maat013-in-patients-with-acute-graft-versus-host-disease/

Luis Gosálbez
Sandwalk BioVentures is a specialty strategy, innovation, regulatory and management consulting firm focused on microbiome technologies servicing companies in the food and pharma sectors, as well as financial and strategic investors exploring to enter this field. The company has created the Microbiome Drug Database™, an online repository containing the most extensive and thorough analysis of biotechnology companies developing pharmaceuticals from or through the microbiome.

