Microbiome-focused drug development is often thought of as the addition of beneficial strains or consortia of bacteria to specific sites in the body. Yet some drug products rather aim to take away elements or profoundly modify their activity at the molecular level—in other words, ‘edit’ the microbiome—in order to achieve a therapeutic effect. In this context, bacteriophages (or “phages”) are increasingly of interest for their ability to selectively remove or alter bacterial members of the microbiome. Here we summarize and provide an analysis of the bacteriophage drug development sector from the Microbiome Drug Database™, the most complete repository of biotechnology companies developing microbiome-derived drugs.
Phage-based drug development was recently brought into the spotlight after BioNTech’s acquisition of the Austrian bacteriophage company Phagomed for a reported sum of €150M [1]. This is not only the first sizeable acquisition in the phage drug development space, but also an endorsement of bacteriophage technology overall, given BioNTech’s leadership in many cutting-edge areas of biopharmaceutical development such as mRNA and CAR-Ts.
-
The basics of bacteriophages
Bacteriophages are viruses that infect bacteria rather than other organisms. So while they are theoretically harmless to humans, they can be lethal for their bacterial targets, which they infect and kill to generate new bacteriophages that will continue the cycle with neighbouring microorganisms of the same type.
After phages were discovered in 1915, they proved to be useful tools in research and helped lay the groundwork for modern molecular biology. For example, phages were used to identify the basis of genetic material, and the finding that 3 nucleotides code for an amino acid. They also allowed the identification of restriction enzymes [2][3]. More recently, the study of the phenomenon of phage resistance in bacteria led to the discovery of the CRISPR-Cas system [4].
However, research on phages as therapeutics remained slow until recently. A better understanding of microbial ecology, better analytic techniques and burgeoning public health challenges have renewed the interest of the scientific community in using bacteriophages as drugs.
Microbiome research in the past decade has focused disproportionately on the bacterial elements of microbial ecosystems. However, new tools and microbiome analysis techniques have shown that bacteriophages play a crucial role in determining the composition and activity of microbial communities. Bacteriophages may in fact be the most abundant organisms in the biosphere, outnumbering other members of the microbiome, such as bacteria, by up to 10-fold [5]. One study identified over 54,000 bacteriophage species in the human gut, 90% of which were previously unknown to scientists [6]. Many scientists and companies are now exploring the potential uses, therapeutically and otherwise, of this previously untapped part of the microbial dark matter.
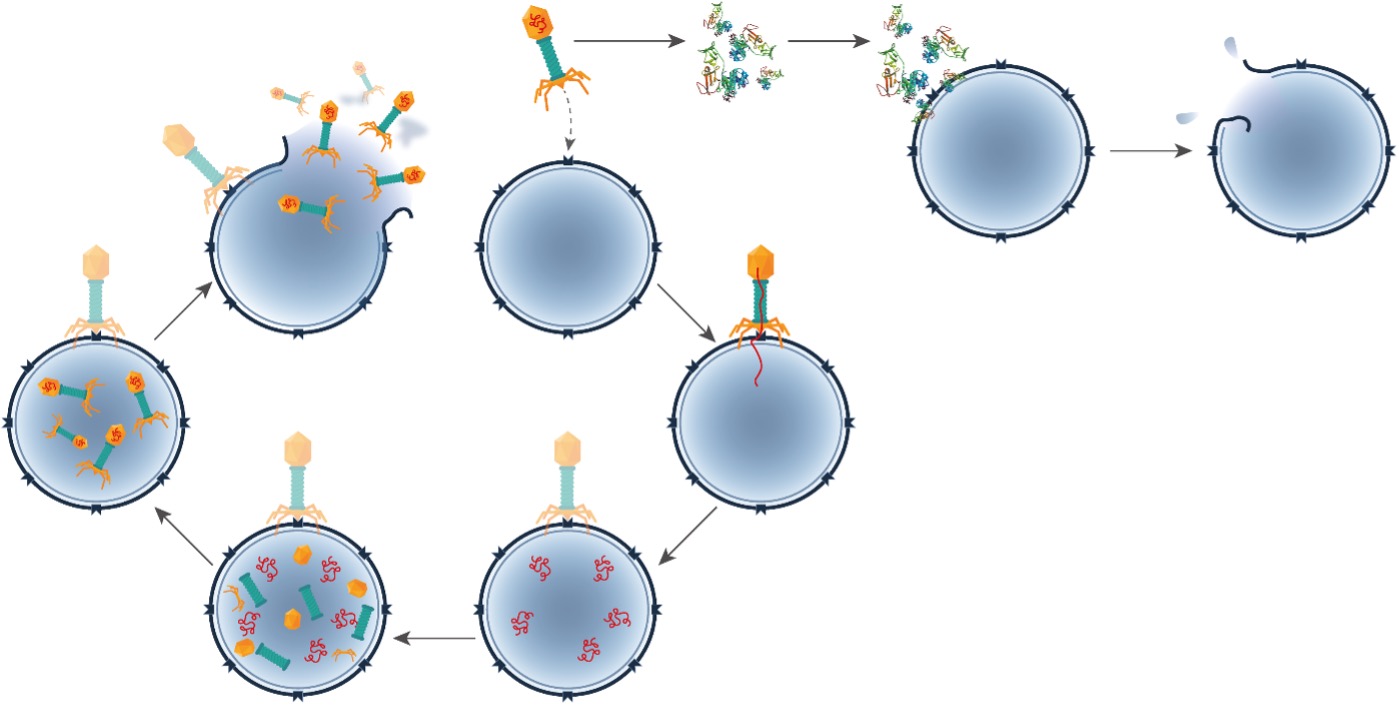
Figure 1. Bacteria-killing system of phages and lysins.
-
Phages as drugs in the biotechnology industry
Treating infectious diseases is a leading application of phages because of their potential specificity for killing selected bacterial organisms. However, using phages for this purpose is in no way a recent idea; as early as 1917 (only two years after the discovery of their existence, and decades before antibiotics were in widespread use) Felix d’Herelle suggested they could be used to kill bacteria [7].
With recent interest in the microbiome, phages have once again received attention for being able to target infectious microbes. As non-chemical, targeted and highly selective agents, they are thus likely to have fewer effects on the microbiome overall, compared to antibiotics.
As biological entities, phages can co-evolve (or be made co-evolve) with their target to overcome antimicrobial resistance. This is of enormous value since, according to the Centers for Disease Control and Prevention (CDC), almost 3 million people in the US acquire an antibiotic-resistant infection each year, and about 30,000 people die. A World Health Organization report in 2020 concluded that 80% of new antibacterial agents approved since 2017 offer little benefit when it comes to antimicrobial resistance as they are derived from existing drugs, against which pathogenic microbes have already developed resistance mechanisms [8]. The COVID-19 pandemic has again put the threat of antibiotic resistance under the spotlight, as many experts have warned that antimicrobial resistance may be the next global health crisis and may claim many more lives. Therefore, finding additional or alternative treatments for infections is regarded as crucial.
For these reasons, the characteristics of phages make them a very promising tool for the treatment of infections, especially those caused by multi-drug resistant microbes. While phages are not poised to take the place of antibiotics, they may gain prominence as an important adjunctive therapy to deal with clinically complex infections.
So how are companies leveraging the potential of phage therapies? An analysis of the Microbiome Drug Database™, the most comprehensive repository of biotechs developing microbiome drugs, reveals that almost 70% of the total of 100 company-sponsored, phage-based drug development programs focus on the treatment of infections. These programs target a range of bacterial infectious agents, such as P. aeruginosa infections in cystic fibrosis patients, K. pneumoniae-mediated pneumonia, urinary tract and systemic infections caused by E. coli, and bacterial sepsis originating from multidrug-resistant S. aureus. These programs use wild-type or engineered phages or phage cocktails for their bacteria-killing capacity.
Infectious diseases, however, are not the only application that companies have found for phages as drugs.
After infectious diseases, dermatology is another major area in which phages have been identified as potentially useful. Most of these programs use topical gels or sprays containing native or engineered bacteriophages against Cutibacterium acnes (formerly known as Propionibacterium acnes) as the purported causative agent of acne. This is in fact an area where one of the most potentially lucrative agreements in microbiome drug development has been signed: the one between the French company Eligo Bioscience and GlaxoSmithKline, worth up to €200M in licensing fees and potential milestone payments and royalties. Phages targeting S. aureus and other bacterial species are also being tested to treat burns susceptible to infections, and atopic dermatitis, where bacteria are thought to contribute to the development and exacerbation of inflammation through the release of virulence factors that affect keratinocytes and skin immune cells.
It is worthwhile to know that for both infectious diseases and dermatology, 21 development programs are employing lysins, the enzymes produced by bacteriophages to break down the bacterial cell wall, as a molecular bacterial-killing strategy. This approach goes beyond phages and fast-tracks to the products they produce. In this so-called molecules from the microbiome / drugs from bugs approach, molecules derived from microbes (lysins), rather than full organisms (phages) are employed as a therapeutic. This approach has a number of advantages over delivering the microorganisms themselves, including regulatory, as will be discussed below. Also, these lysins may be engineered (with some companies already taking this approach) to improve their specificity or efficacy in eradicating different microbes.
Immuno-oncology is in fact the therapeutic area of fastest growth for phage-based therapies over the past several years. Currently, almost 20% of the development programs focus on this segment, with an almost 300% increase compared over the last 2 years. Analogously to infectious diseases, phages are being tested as an adjunctive therapy to the existing standard-of-care to either kill cancer-promoting bacteria (for instance gut microbes in colorectal malignancies) or to locally deliver the genes for the expression of tumor-associated antigens to prime the immune system and trigger immunity.
Phages are also being employed to modulate the microbiome and eliminate, or edit out, bacteria that have been related to the development and progression of immune-mediated diseases such as Inflammatory Bowel Disease (IBD).
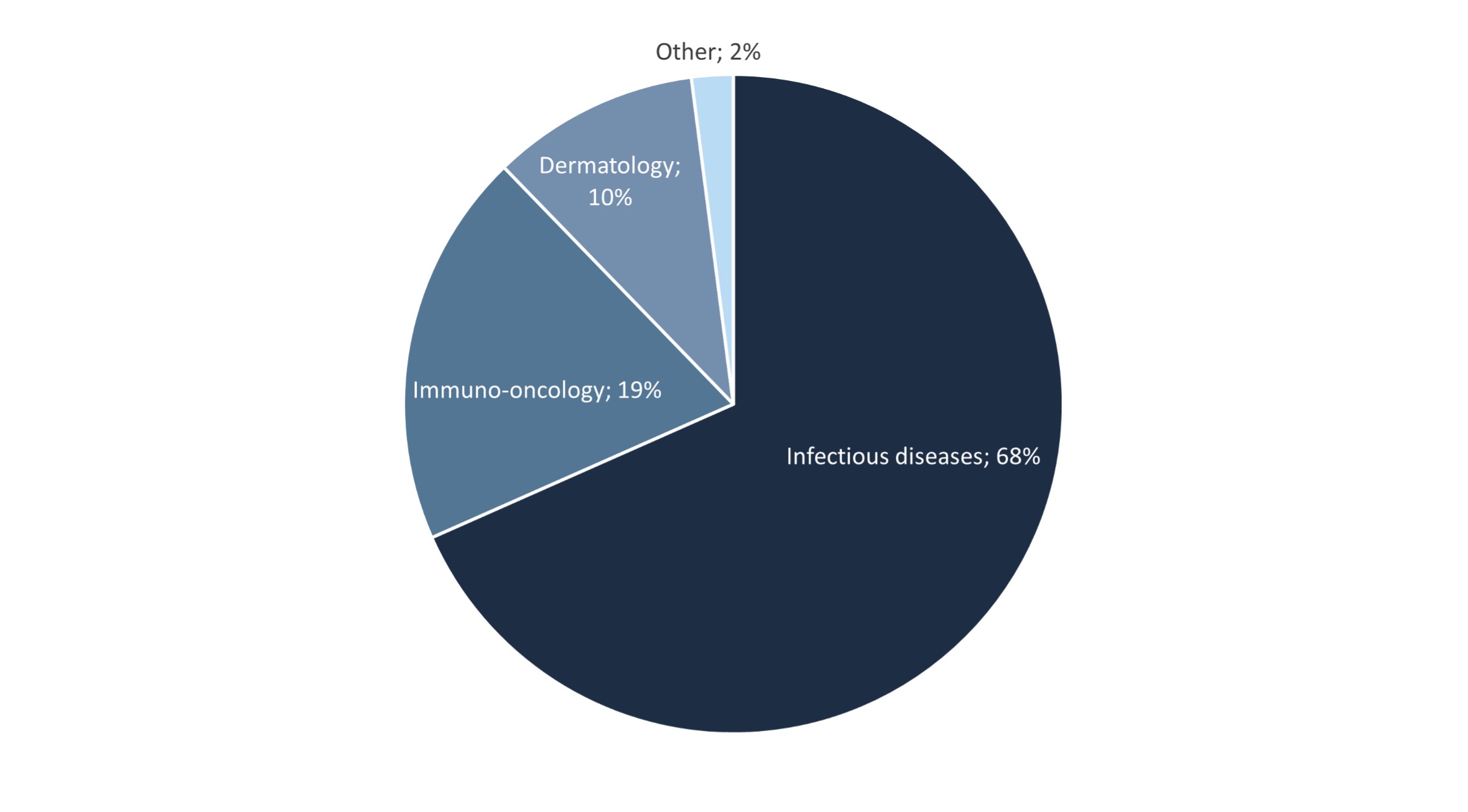
Figure 2. Therapeutic applications of phage-based drugs in the industry (includes phages and lysins)
Interestingly, a new application for bacteriophages has been recently developed: COVID-19 vaccines based on engineered phage particles. These programs employ phages as delivery vehicles for the expression of SARS-CoV-2 spike protein to confer immunity, similar to the mRNA vaccines broadly utilized to tackle the pandemic worldwide.
-
More companies interested in phage-based therapies
In addition to the expansion in the therapeutic applications of phages described above, a comparative analysis of the Microbiome Drug Database™ between the years 2019 and 2021 shows that the bacteriophage drug development sector is certainly growing and developing, with more companies taking on the scientific and technical challenges of developing phages as drug products.
There are currently 30 different companies engaged in the development of phages and/or lysins as drugs, up 60% from 2019. The number of development programs these companies are engaging in has also increased in just 2 years (100 ongoing today vs. 52 in 2019). All of this growth has been accompanied by significant economic investment, with almost €400M in funding during this period. Funding for companies engaged in phage-based drug development has been close to €1.2 billion to date by our records.
Another useful metric to describe the development of this segment is the development stage for the majority of the research programs: there are already 25 industry-sponsored, phage-technology-based development programs in clinical phase.
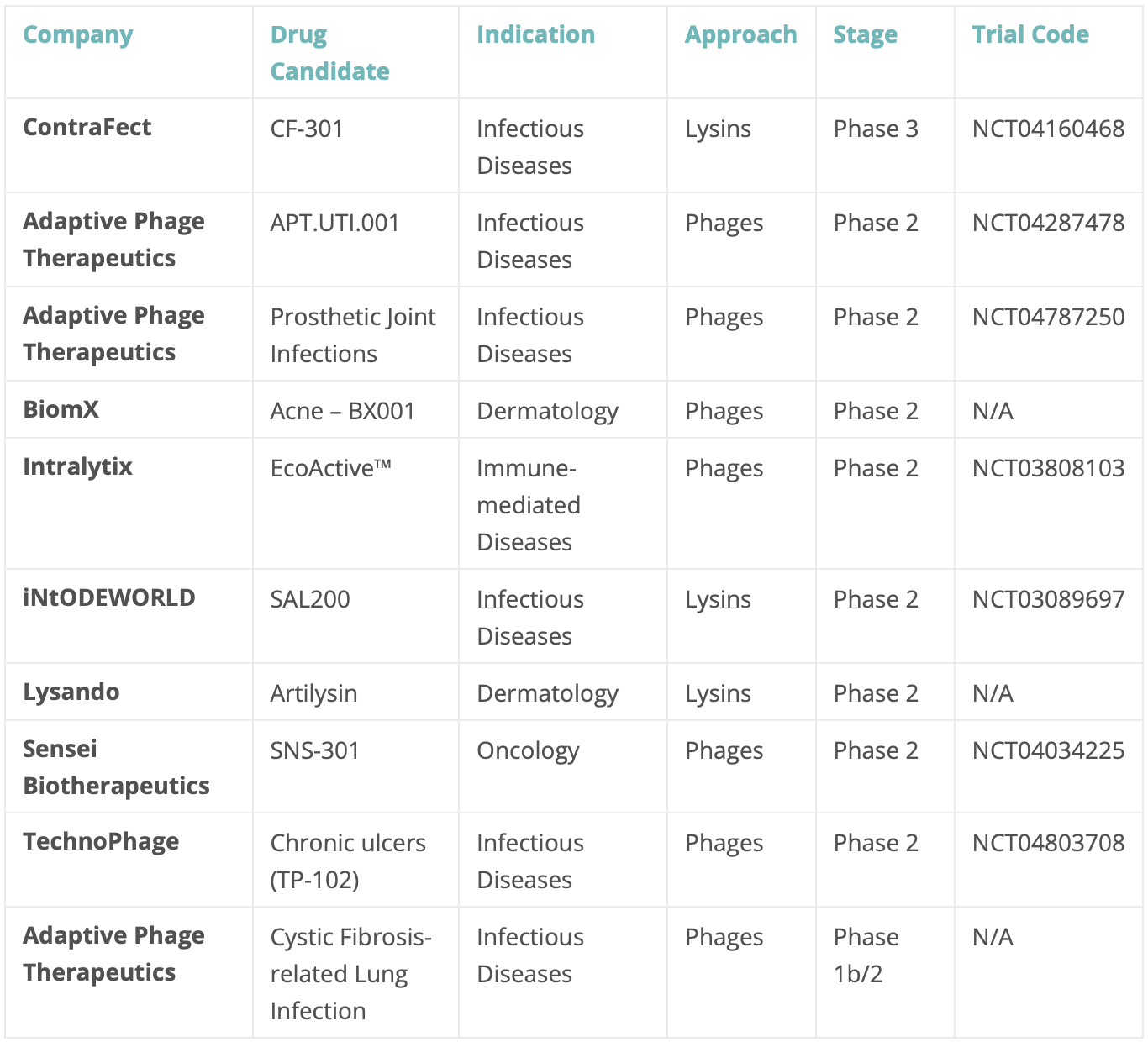
The only company-led program that has reached Phase 3 in this segment is Contrafect’s CF-301 (NCT04160468), which employs a recombinantly-produced bacterial lysin (exebacase) for the treatment of S. aureus bloodstream infections, including endocarditis. Whereas no full-phage, industry-led clinical program has reached Phase 3, there are already 10 programs in Phase 1 (up 250% from 2019) and 7 in Phase 2 (up 133% from 2019, see Table below).
Table 1. Late-stage, phage-based, industry-sponsored clinical pipeline
-
Room for hope in regulatory affairs
Regulations—or more specifically, the lack of specific regulations for phage-based therapies—is regarded by those in the industry as a major challenge.
There are currently no phage-based drugs in Europe or the US, with the exception of some phage-based drugs approved many years ago in certain eastern European countries. Phage therapies are indeed being used in many countries around the globe, but on the basis of compassionate / emergency use to treat individual patients as a last resort for treatment resistant bacterial infections, and usually paired with prior phage susceptibility assays. In the US, this use is set out in the Food and Drug Administration’s (FDA’s) Expanded Access pathway [9]. In Europe, this tool is protected by Article 83 of Regulation (EC) No 726/2004, but is coordinated and implemented by individual Member States. However potentially life-saving phage therapies may be in this context, their use is complex, anecdotal and not scalable. The interest of biotechnology and pharmaceutical companies is finding a clear pathway for the development of these therapies in a standardized manner.
In Europe, the European Medicines Agency (EMA) has acknowledged that none of the regulations currently fit bacteriophage therapy adequately, although its opinion is that the existing pharmaceutical regulatory framework is adequate for evaluating its potential benefits and risks. This has been ratified by the European Commission in an official statement [10][11]. EMA’s recommendation is twofold: first, that additional clinical trials are initiated to further document how bacteriophages should best be applied in clinical practice, and second, that developers and sponsors should engage with the Agency in an early dialogue on how to develop these medicinal products in the context of the existing pharmaceutical legal framework [12][13].
For insights into the European approach for regulating some phage-based therapies, very recently (2021), the EMA requested a scientific recommendation on the classification of a drug under development based on bacteriophage particles carrying recombinant DNA encoding CRISPR/Cas circuits that enable specific killing of E. coli for the treatment of bloodstream infections in neutropenic patients with hematological malignancy. The Agency concluded that this product should follow the Gene Therapy Medicinal Product pathway based on the recombinant nucleic acid content of the active substance and the direct relation between the expression of this genetic content and the therapeutic effect of the drug.
In the US, the situation is similar, with a lack of specific regulations and existence of some non-binding guidance for industry. Yet it is different in practice because, as mentioned above, multiple clinical trials with bacteriophages have been authorized by the FDA so far.
The situation is different, and more optimistic, for lysins. Being isolated, relatively small molecules, which may be more easily characterized from a biochemical standpoint, and without the capacity to replicate, their regulatory pathway and CMC matters may be more straightforward.
Outside the realm of drugs, we find an analogous discrepancy between the US and Europe, and also some room for hope. In the US, since 2006 the FDA has issued a total of 14 No-objection letters to Generally-Recognized As Safe (GRAS) applications for bacteriophages, and one for a lysin, as food ingredients. These applications concerned preparations to preserve food safety against Listeria, Salmonella, Shigella, E. coli, C. jejuni and C. perfringens. Although this procedure goes through a completely different branch of the FDA, it proves that there may indeed be a positive view towards the use of phages in humans in the US. Other bacteriophages with different food applications, including purported health-promoting / functional properties, are being marketed on a self-affirmed GRAS basis.
In Europe, the European Food Safety Authority issued its first-ever scientific opinion on a bacteriophage in early 2021 [15], regarding a feed additive consisting of four bacteriophages against Salmonella gallinarum for farm avian species. Although the opinion of the Authority was not completely positive due to their assessment that the data to back its efficacy is insufficient (as proof of efficacy is compulsory to gain the zootechnical additive status in the EU), EFSA expressed the view that the product does not pose a risk for the animals, the consumers or the environment. Again, EFSA is not the authority deciding on the safety or efficacy of ingredients in drugs, this product was not aimed for direct human consumption (or intravenous administration) and the overall outcome was not completely positive, but this, together with the EMA’s opinions stated above, shows that phages are being considered by European authorities.
-
Conclusions and future outlook
Phages offer the potential for highly-specific ‘editing’ of microbiomes. Recent progress related to phages, as described above, establish bacteriophages as one of the multiple exciting scientific frontiers in microbiome research—and in particular, a promising approach for therapeutic development. Phages are remarkably simpler than other elements of the microbiome from a biological standpoint, which may make their development as pharmaceuticals significantly easier.
As clinical validation becomes more solid and regulatory affairs become clearer, there will be growing interest from larger corporations in using phages and their molecules as drugs.
References
[1] https://www.sandwalkbio.com/post/biontech-acquires-bacteriophage-company-phagomed
[2] https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3109452
[3] https://link.springer.com/book/10.1007/978-1-60327-164-6
[4] https://link.springer.com/article/10.1007%2Fs00239-004-0046-3
[5] https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3109452
[6] https://www.nature.com/articles/s41564-021-00928-6
[7] https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3109452
[8] https://www.who.int/publications/i/item/9789240021303
[9] https://www.fda.gov/news-events/public-health-focus/expanded-access
[10] https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5812529
[11] https://www.europarl.europa.eu/doceo/document/E-8-2015-013488-ASW_EN.html
[12] https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5812529
[13] https://www.ema.europa.eu/en/documents/other/workshop-therapeutic-use-bacteriophages-summary_en.pdf
[14] https://www.clinicaltrialsregister.eu
[15] https://www.efsa.europa.eu/en/efsajournal/pub/6534

Luis Gosálbez
Sandwalk BioVentures is a specialty strategy, innovation, regulatory and management consulting firm focused on microbiome technologies servicing companies in the food and pharma sectors, as well as financial and strategic investors exploring to enter this field. The company has created the Microbiome Drug Database™, an online repository containing the most extensive and thorough analysis of biotechnology companies developing pharmaceuticals from or through the microbiome.


