
The development of novel oral therapeutics—such as live biotherapeutics, peptides, proteins, and nucleotides—has introduced new challenges in drug formulation and delivery. In this article, we describe Evonik’s solution – EUDRACAP® colon – a ready-to-fill, functional capsule designed to address some of these challenges.
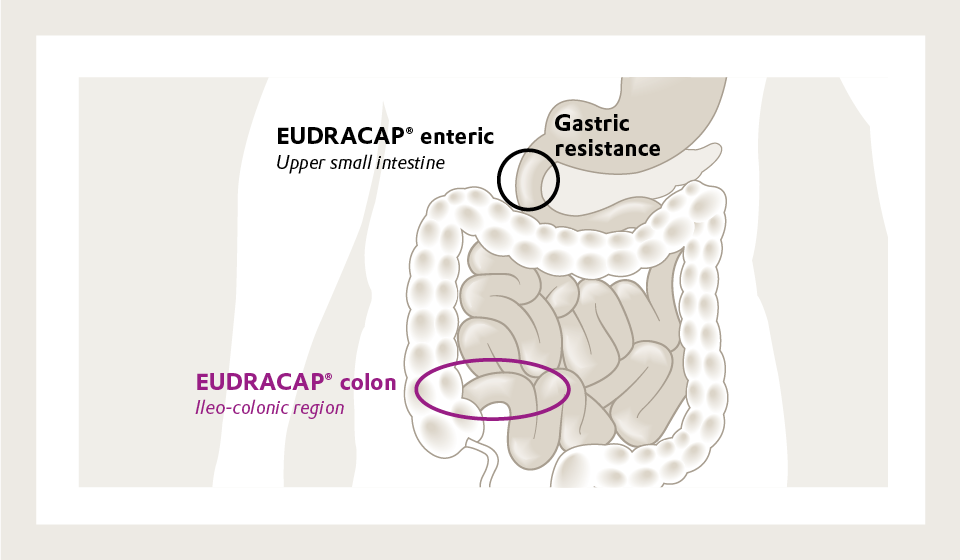
Many novel active pharmaceutical ingredients (APIs) are highly sensitive to environmental conditions and are unstable in acidic environments or susceptible to enzymatic degradation. Conventional oral dosage forms such as uncoated capsules or tablets may not provide adequate protection, leading to reduced bioavailability or loss of therapeutic activity. These emerging classes of APIs often also require precise delivery to specific regions of the gastrointestinal (GI) tract. The ileo-colonic region is of particular interest due to its role in local and systemic drug absorption, as well as its relevance in microbiome-targeted therapies.
EUDRACAP® colon is designed to protect sensitive APIs during manufacturing and transit through the upper GI tract, releasing its contents only upon reaching the desired site. The capsule incorporates a pH-dependent release mechanism enabling it to remain intact in the stomach and upper small intestine. This targeted release profile is achieved using EUDRAGIT® polymers, which are widely known as pharmaceutical coatings for their well-characterized solubility behavior.
Figure 1. Comparing the regions for drug release for EUDRACAP® colon and EUDRACAP® enteric
Mechanism of Action and Dissolution Profile
The performance of EUDRACAP® colon has been validated through in vitro dissolution testing using caffeine as a model compound. Results demonstrate that EUDRACAP® colon exhibits no release during the acid stage (0.1N HCl) or at pH 6.8. Release begins only upon exposure to pH 7.2, confirming its suitability for ileo-colonic targeting. In contrast, EUDRACAP® enteric—a related capsule designed for upper intestinal release—initiates release at pH 6.8 (see Figure 2).
Figure 2 Comparing dissolution profiles of EUDRACAP® colon and EUDRACAP® enteric
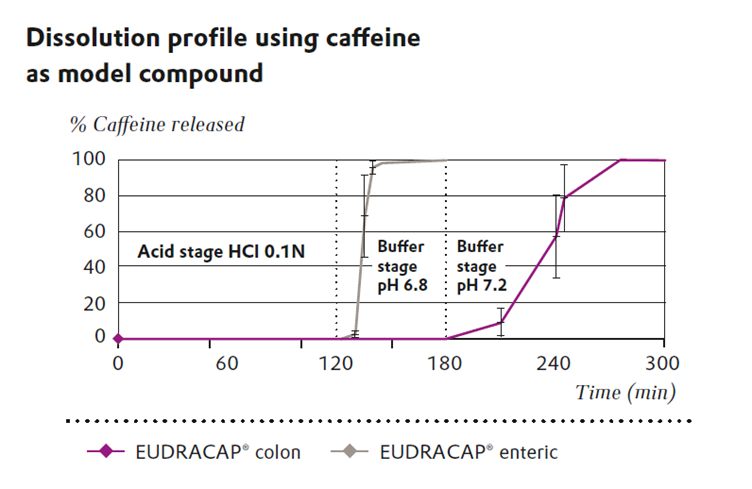
This delayed release profile is particularly advantageous for APIs that require protection from gastric acid or enzymatic degradation, or for those intended to act locally in the colon, such as in the treatment of inflammatory bowel diseases or microbiome modulation.
Enhancing Treatment Efficiency and Patient Compliance
Targeted delivery to the ileo-colonic region offers several therapeutic advantages. For localized treatments, such as those aimed at modulating the gut microbiome or treating inflammatory bowel diseases, precise delivery ensures that the drug acts directly at the site of disease, enhancing efficacy and minimizing systemic side effects.
For systemic therapies involving peptides, proteins, or other biologics, the ileo-colonic region provides a favorable environment for absorption. By delivering the API to this region, it is often possible to reduce the required dosage, which can lead to fewer side effects and improved patient compliance.
Moreover, the ability to use a single, functionalized capsule for both protection and targeted delivery simplifies the patient experience. Instead of multiple pills or complex dosing regimens, patients can take a single capsule that delivers the medication exactly where it is needed.
Design Considerations and Manufacturing Compatibility
EUDRACAP® colon is based on hydroxypropyl methylcellulose (HPMC) capsules, which offer high stability and compatibility with a broad range of APIs. The capsules are pre-closed and coated using a proprietary process that ensures uniformity and integrity of the functional layer. This design eliminates the need for additional formulation steps or capsule banding to seal the cap-body interface.
The capsules are compatible with standard capsule-filling equipment, allowing for seamless integration into existing manufacturing workflows. This reduces development time and complexity, particularly in early-stage clinical programs where speed and flexibility are critical.
Technical Innovation and Validation
One of the key technical challenges in developing a functional capsule for ileo-colonic delivery is achieving a coating thickness that ensures acid resistance without compromising capsule integrity or fillability. The coating must also maintain a tight seal after filling to prevent leakage or premature release.
The coating process developed for EUDRACAP® colon addresses these requirements by applying a robust functional layer to pre-closed capsules. This ensures consistent performance across batches and simplifies the handling of sensitive APIs during manufacturing.
Supporting the Future of Pharmaceutical Innovation
As the pharmaceutical industry continues to explore novel therapeutic modalities, the need for advanced drug delivery systems is becoming increasingly evident. EUDRACAP® colon provides a scientifically validated, manufacturing-ready solution for the targeted delivery of sensitive APIs to the ileo-colonic region.
Its design supports both local and systemic therapeutic strategies, offering a versatile platform for the development of next-generation oral drugs. By addressing key formulation challenges, this capsule technology enables faster progression from development to clinical evaluation, ultimately supporting the delivery of innovative treatments to patients.
