

Heloise Breton, PhD
Director of Product Management
Sample Management Solutions
The women’s health gap refers to disparities in health outcomes and the limited research into conditions that exclusively affect women or affect them differently from men. This gap stems from the historic exclusion of females from clinical trials (up to 1993) and a long-standing underinvestment in women’s health research and development.1
Within the microbiome field, the vaginal microbiome has garnered particular attention in women’s health research due to its distinct role in female physiology and its connection to a wide range of health conditions and diseases. This dynamic ecosystem of microorganisms undergoes significant shifts throughout a woman’s lifespan, influenced by factors such as menstruation, menopause, and hormonal changes.
A healthy vaginal microbiome is typically dominated by Lactobacillus species. These bacteria secrete antimicrobial compounds that help maintain microbial balance and protect against infection. When this balance is disrupted, it can lead to conditions such as bacterial vaginosis, urinary tract infections, pre-term birth, and increased susceptibility to sexually transmitted infections.2
Several companies are investigating ways to better understand—and potentially target—the vaginal microbiome including vaginally applied probiotics, oral probiotics, and consumer test kits for health monitoring.
Women’s Health Research Requires Robust Tools
To develop meaningful interventions, the vaginal microbiome must be studied using reliable and replicable methods. Today, next-generation sequencing (NGS) is the standard tool for characterizing its complexity. However, each step of the NGS workflow — from sample collection to data analysis — can introduce bias. Establishing robust standards is therefore essential to ensure accuracy and reproducibility across studies.3
Methodology studies have already shown the importance of sample collection and nucleic acid extraction protocols in microbiome studies. Suboptimality in both steps can potentially introduce a significant bias in resulting microbial profiles.
Sample Collection
Robust tools for collecting and stabilizing vaginal microbiome samples are essential to ensure that what’s analyzed in the lab accurately reflects the in vivo state. Without proper stabilization, the integrity of the sample can degrade, compromising the reliability and relevance of study results. Typically, vaginal samples are collected using a swab which can be stabilized at the time of collection through freezing or a stabilizing buffer to retain its biological make-up.
Nucleic Acid Extraction
An appropriate extraction protocol must lyse gram-positive and gram-negative bacteria, support optimal recovery of the nucleic acids and mitigate the introduction of contaminants, e.g. bioburden.
The OMNIgene™•VAGINAL Device Captures an Accurate Representation of the in vivo Microbiome
Recognizing the importance of sample collection and extraction stages in vaginal microbiome studies, DNA Genotek Inc. has developed the OMNIgene™•VAGINAL microbial collection device, as well as the OMNIgene™•XTRACT ULTRA microbial extraction kit, to enable replicable robust science.
The OMNIgene™• VAGINAL Device
The OMNIgene™• VAGINAL device is a microbial collection device scientifically proven to collect and stabilize microbial DNA and RNA for molecular-based applications. The device eliminates bias introduced by microbial overgrowth and decay by halting the biological activity of the sample upon collection.
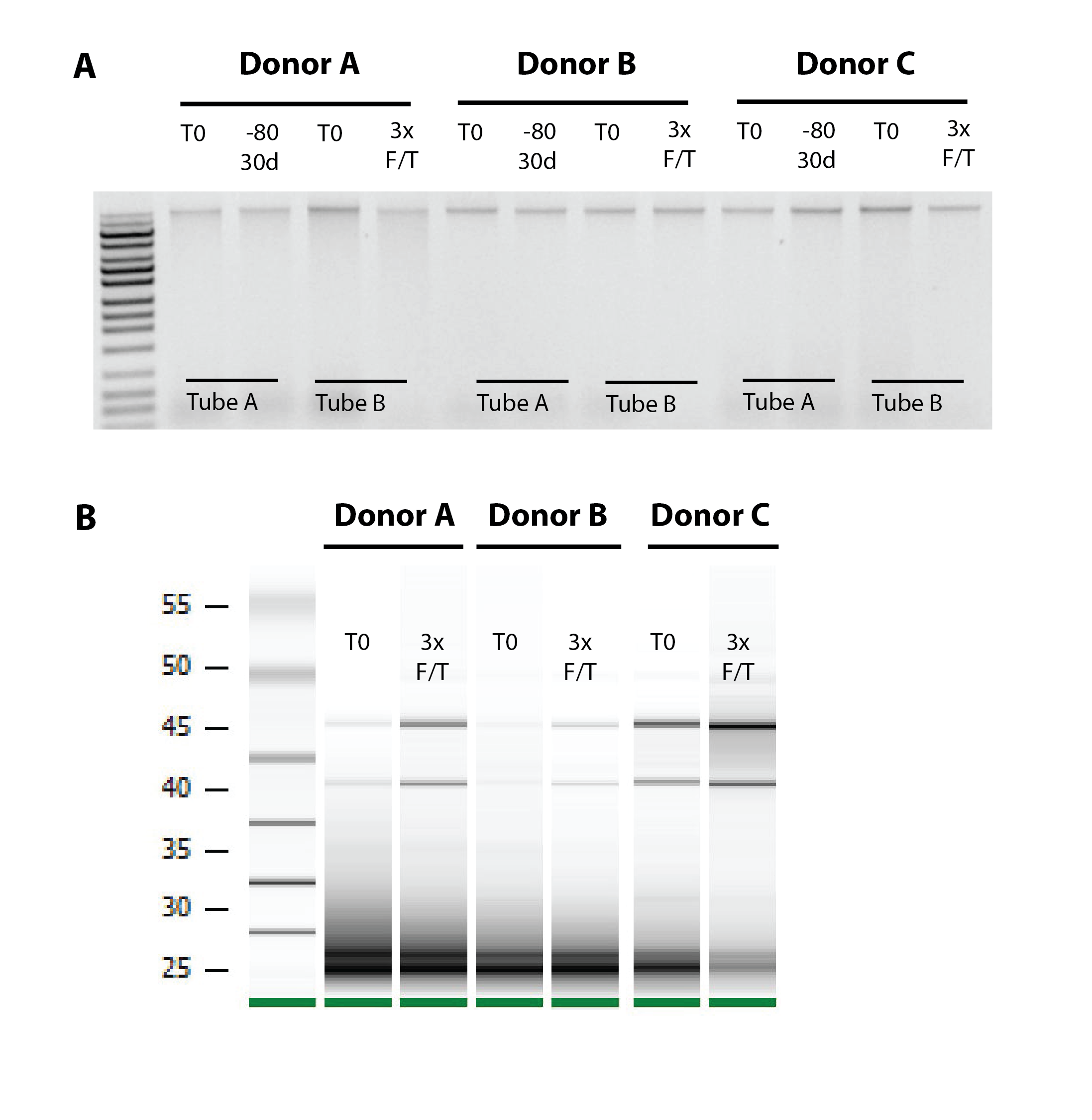
Whilst some sample collection protocols require instant freezing, introducing a financially burdensome step that can present logistical barriers to field studies, the OMNIgene™• VAGINAL device enables self-collected samples to withstand room temperature for up to 30 days. In fact, the device has even been demonstrated to preserve DNA and RNA stability in harsh conditions, with vaginal DNA and RNA stability being retained following 3 freeze-thaw cycles – a treatment known to be highly detrimental to nucleic acid stability (see Figure 1).4
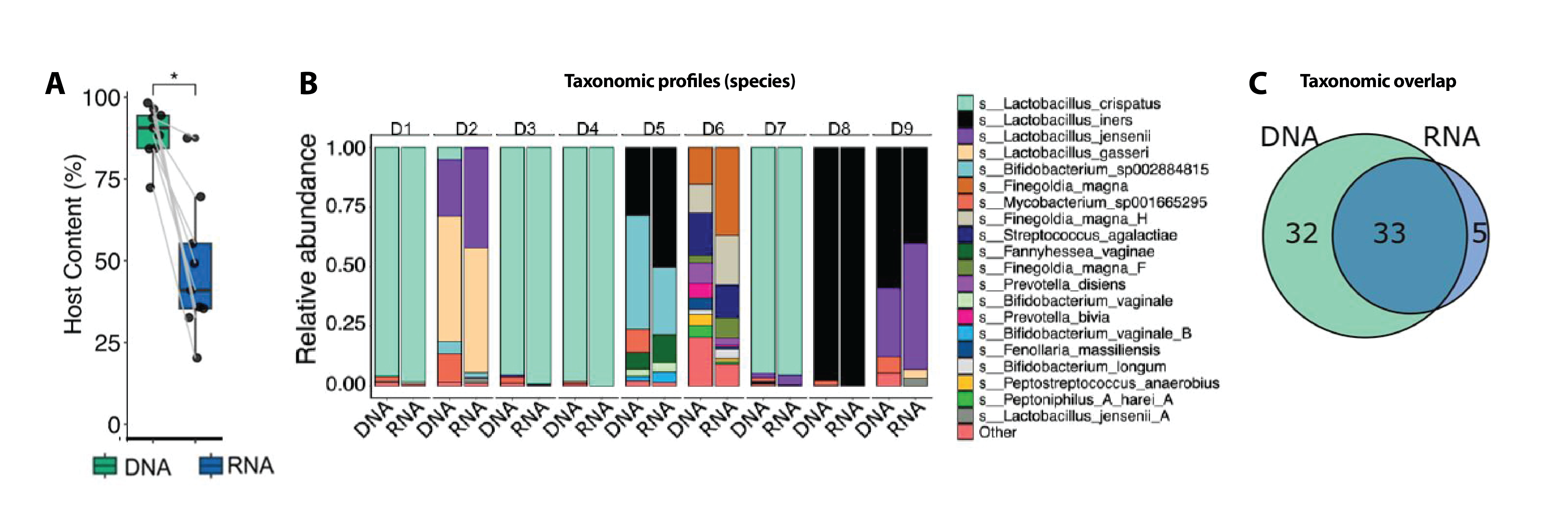
Samples collected in the OMNIgene™•VAGINAL device have also been shown to support metagenomic and metatranscriptomic sequencing, with taxonomic profiles derived from each analyte generally correlating – indicating the collection device reliably preserves the core microbial community, giving reproducible profiles across analytes (Figure 2).5
The OMNIgene™•XTRACT ULTRA Microbial Extraction Kit
The OMNIgene™•XTRACT ULTRA extraction kit is a nucleic acid extraction technology that enables improved efficiency of nucleic acid extraction, optimized for use with OMNIgene™ collection kits, including the OMNIgene™•VAGINAL device.
Most extraction kits are optimized to process unstabilised primary microbiome samples, and compatibility is not guaranteed when using kits optimized to process unstabilised microbiome samples on stabilized ones – often leading to poor recovery of DNA and RNA.
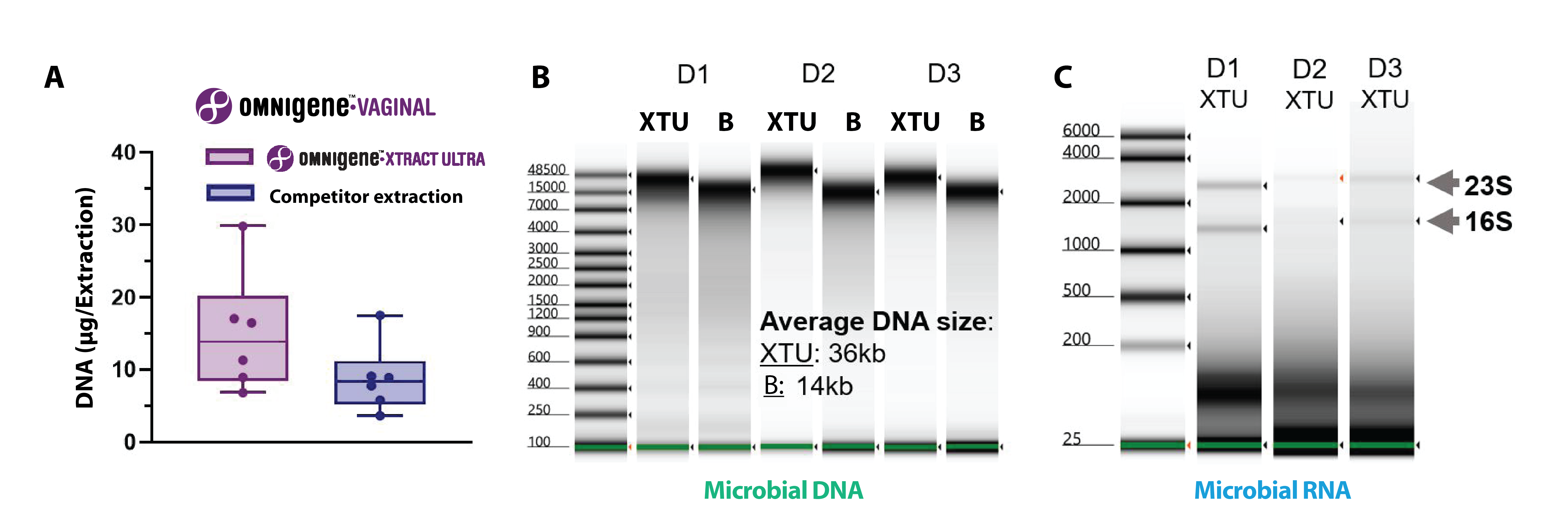
The OMNIgene™•XTRACT ULTRA extraction kit overcomes extraction challenges by providing unparalleled extraction performance on vaginal microbiome samples, improving nucleic acid yield and analyte quality compared to other devices on the market.
In one study, the OMNIgene™•XTRACT ULTRA extraction kit’s performance was compared to a leading competitor kit. The results showed the OMNIgene™•XTRACT ULTRA extraction kit recovered higher quantities of high-quality DNA and RNA from vaginal microbiome samples, with average DNA fragment sizes of >30 kb for vaginal samples compared to 14 kb for the competitor kit (Figure 3.)5
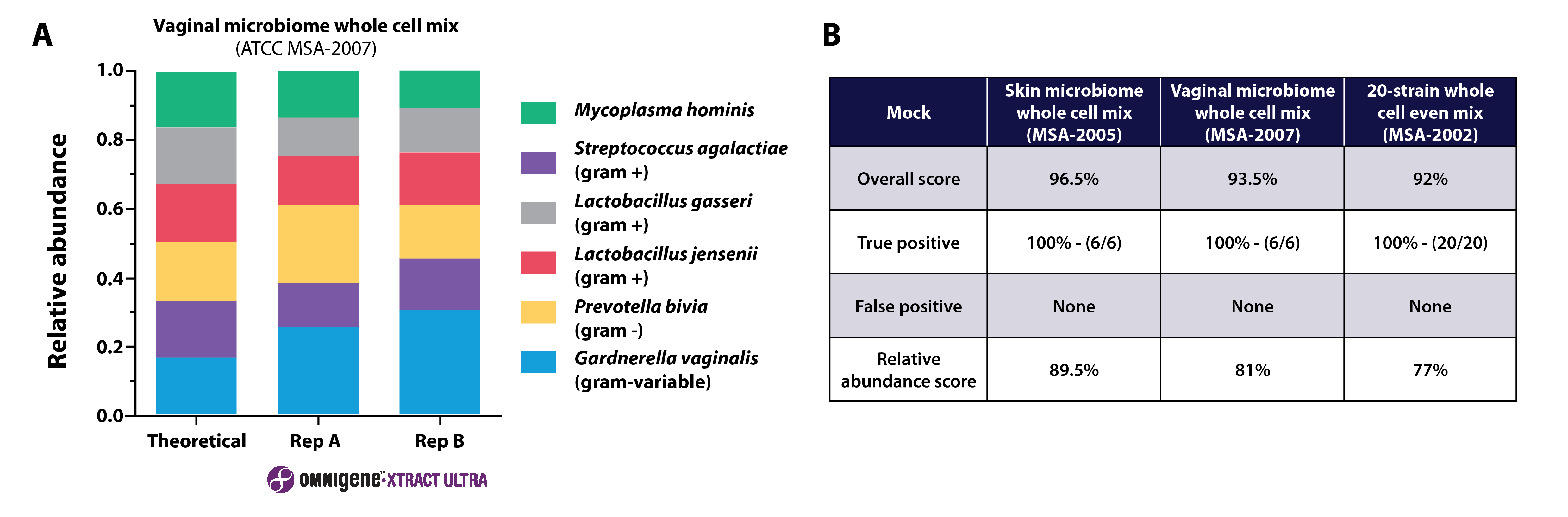
Moreover, the OMNIgene™•XTRACT ULTRA extraction kit has been optimized for vaginal samples, demonstrating efficient lysis of both gram-negative and gram-positive bacteria and accurately captured vaginal-specific taxonomic profiles. Analysis of a vaginal mock community treated with the OMNIgene™•XTRACT ULTRA extraction kit protocol showed no false positives, and a 93.5% similarity to the theoretical composition (Figure 4).5
Additionally, the OMNIgene™•XTRACT ULTRA extraction kit technology does not contain or require the use of hazardous chemicals such as guanidinium salts or phenol-chloroform, increasing user safety and minimizing the generation of chemical waste.
The solution enabling important Women’s Health research
Featuring in numerous publications, the robust performance and easy-to-use nature of the OMNIgene™•VAGINAL collection device has led it to be an obvious choice for microbiome researchers looking to unravel the contributions of the vaginal microbiome to women’s health.6,7,8
Looking to learn how the OMNIgene™•VAGINAL device can make your microbiome research more replicable? Visit here.
Sources:
- https://orwh.od.nih.gov/toolkit/recruitment/history
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8058480/
- https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2023.1094800/full
- https://www.dnagenotek.com/row/pdf/PD-WP-00076.pdf
- https://dnagenotek.showpad.com/share/cwKhtdkJKB6V3QM9ovJ7N
- https://onlinelibrary.wiley.com/doi/full/10.1111/apm.13261
- https://link.springer.com/article/10.1186/s12879-024-10313-3
- https://link.springer.com/article/10.1186/s12889-024-20491-z
