
In the past 10 years, there has been significant interest in new classes of active pharmaceutical ingredients (APIs). These novel APIs include live biotherapeutics, which are used for microbiome targeting drugs, oral biologics like GLP1 agonists and nucleotides such as RNA. Innovative APIs like these are being developed to fight diseases that affect a large number of people worldwide, including diabetes, obesity, Parkinson’s disease, Alzheimer’s disease, cancer and bowel diseases like ulcerative colitis and Crohn’s disease.
Developing drugs to treat many of the diseases listed above requires delivering the active ingredient to the final section of the small intestine, or rather the beginning of the colon known as the ileo-colonic region. To target this region most efficiently, a delivery method specifically for the ileo-colonic region is needed.
Many novel APIs are very sensitive to acid, heat, moisture or enzymes. This means that it is not possible to formulate these sensitive APIs into tablets or uncoated capsules because the process conditions would damage or degrade the API. Protection of the API is therefore needed both while formulating the API and for targeted delivery.
In response to this trend and to address these challenges, Evonik has developed a pre-coated, ready-to-fill capsule to specifically target the ileo-colonic region.
In this interview, Dr. Bettina Hölzer, Global Product Manager EUDRACAP® at Evonik, gives further insights into EUDRACAP® colon and the role it plays in bringing novel oral drugs to market faster.
Q: Why did Evonik develop EUDRACAP® colon?
A: We are, of course, constantly looking at trends in R&D, and the pharmaceutical market. There are currently more than 700 drug development programs that use innovative active ingredients. In fact, these new drug candidates make up around a third of all new oral drug developments in the pipeline. We observed that there was a need for a capsule that could be used for sensitive APIs, and also that there were an increasing number of indications requiring ileo-colonic targeting. We already have a portfolio of ready-to-fill coated capsules targeting enteric release, and a 70-year history of targeted oral drug delivery with EUDRAGIT® polymers. Leveraging our expertise to develop a capsule for the ileo-colonic region was the next logical step.
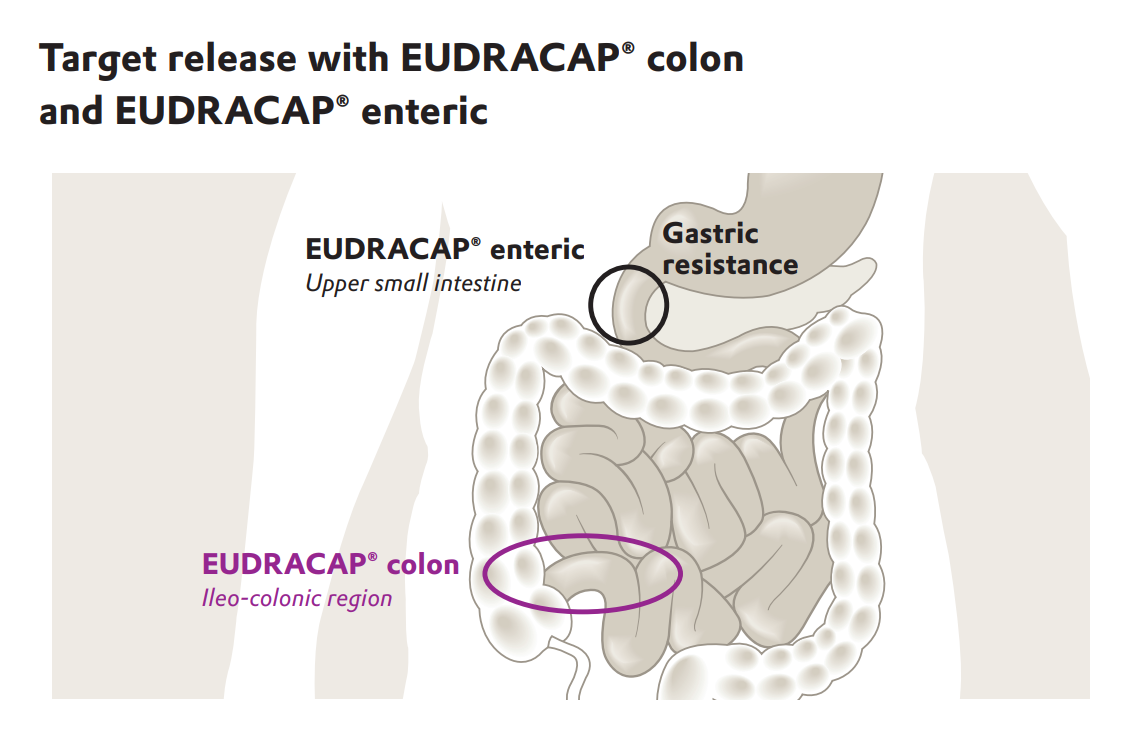
Q: Why is it important to deliver medications specifically to the ileo-colonic region of the gastrointestinal tract?
A: Targeting the ileo-colonic region is important because it enhances the treatment efficiency of medications. This is particularly true for localized therapies aimed at the gastrointestinal microbiome and for systemic absorption of peptides and proteins. But many novel APIs also require targeting to this region. By targeting delivery, the quantity of API used can often be reduced which can mean that patients need to take fewer doses. This can help improve patient compliance and also reduce any adverse effects associated with non-specific drug distribution.
Q: What sets EUDRACAP® colon apart from other functional capsules on the market?
A: At the moment, EUDRACAP® colon is the only ready-to-fill capsule on the market to target the ileo-colonic region. What makes it unique is a combination of features and functionalities.
First of all, the capsule protects sensitive actives during the manufacturing process of the dosage form. The capsules can be used as functionalized ‘containers’ and this means that there is no need for complex formulation development. This helps to accelerate the overall process and enable quicker entry to clinical trials. But this is not all, EUDRACAP® colon also protects sensitive APIs against the acidic environment of the GI-tract and ensures that it can be transported to the ileo-colonic region. This is another important benefit.
By using EUDRAGIT® polymers in the coating, we ensure that the API is released at the higher pH needed for the ileo-colonic region. High-quality HPMC capsules are used as a basis and these can be filled on common automated capsule filling equipment. No capsule branding is required to close the gap between the capsule cap and body.
Q: Which challenges did you face when developing EUDRACAP® colon?
A: The main challenge for functionalizing empty hard capsules is to apply a sufficient thickness of the coating layer to provide the desired protection. It is crucial to strike a balance so that the capsule is easy to open, fill and close, however, the capsule gap must also be tightly sealed after filling.
We developed an innovative technical solution that allows the application of sufficient functional coating to the pre-closed hard capsule. The resulting functionalized capsule provides reliable acid resistance and release in the ileo-colonic region, which is also ensured by a tight capsule gap after final closing of the filled capsule.
Q: In what ways does EUDRACAP® colon help streamline the drug development process?
A: By using a functionalized, ready-to-fill capsule, it is possible to simplify and streamline the drug development process. There is no need for extensive formulation development which means that complexity, time and risk are reduced. This makes it quicker to enter clinical trials and ultimately commercialize new therapies that can reach patients more quickly.
Q: Looking ahead, what role will EUDRACAP® colon play in the future pharmaceutical landscape?
A: EUDRACAP® colon allows the development of new breakthrough drugs using novel classes of APIs by solving the formulation challenges. As the demand for novel classes of APIs continues to rise, we believe our product is uniquely positioned to support the development of innovative therapies. We are excited to see how EUDRACAP® colon will help advance drug delivery systems in the coming years.
To learn more about EUDRACAP® colon get in touch!
