
Microbiome Times got in touch with Dr Edward Green, CEO of CHAIN Biotechology Ltd – a UK based microbiome Company focused on the development and commercialization of microbial technology for the production and delivery of biotherapeutics.
Dr Edward Green has pioneered technical improvements in microbial strain improvement and fermentation process development for industrial biotechnology applications over the past 20 years, contributing to numerous scientific publications and patents.
In 2014, he founded CHAIN Biotechnology to exploit the therapeutic potential of Clostridia bacteria found in the human gut microbiome to develop a proprietary microbial platform for drug delivery. The platform builds on core engineering skills and involves the development and use of engineered Clostridia to deliver specific therapeutics to the gut. The Clostridium Assisted Drug Delivery (CADD™) platform is highly differentiated and supports a therapeutic product pipeline for a wide range of clinical indications including localized infection, inflammation and CNS signalling.

MT: What are the discoveries that lead up to your current work?
I have spent most of my career working with Clostridia (spore forming anaerobic bacteria). For my first company Green Biologics, we developed and commercialized an advanced fermentation process to produce chemicals.
Clostridia also play a key role in digestion and health in the gut microbiota and CHAIN was formed to develop Clostridia for high value therapeutic applications building on our expertise in microbiology but also our ability to engineer Clostridium using cutting edge molecular biology tools.
At CHAIN, we have developed an efficient fermentation method to obtain high yields of spores, and demonstrated spore germination, cell growth and product formation gut models. In pre-clinical tests, we have demonstrated a dose dependent effect of our target compound in treating inflammation. These discoveries validate our Clostridium-assisted drug delivery (CADD) platform technology for bioactive production and delivery to the gut.
MT: How accurate is your data & how sure are you of your conclusions?
Consistent efficacy data and strain performance data have been generated from multiple in vitro and in vivo studies. The next stage is to generate human data.
MT: How did you feel when you first saw the positive outcome of your research and what is it that gained others’ interest?
We were surprised to see robust strain performance in an anaerobic bacterium. Key attributes of the organism are fast growth, a broad substrate range, and good sporulation and germination efficiencies.
CHAIN’s approach is novel, robust and highly differentiated compared to other microbiome therapeutics that focus on the use of non-engineered bacteria or bacterial cocktails. This means it is more targeted with better understanding on the mechanism of action. In addition, our engineered platform approach supports a therapeutic product pipeline targeting many clinical indications.
MT: What do you expect to be the future applications of your findings?
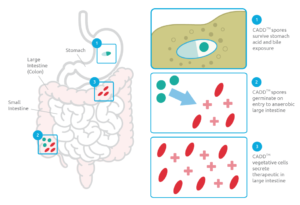
We aim to develop superior therapeutics for gut related and metabolic diseases. There is broad applicability ranging from IBD to CNS and infection. The key advantage of CADD is targeted and sustained gut delivery. The use of spores overcomes degradation in the stomach and upper GI tract. In addition, spores are inexpensive to manufacture and robust, negating the need for cold chain logistics. This CHAIN’s live biotherapeutics particularly suitable for developing countries.
MT: What is needed to turn your findings to true benefit for your company?
The next stage for our lead anti-inflammatory product CHN-1 is to generate clinical data. In addition to our proprietary products, we also plan to develop more exemplar products with biotech companies that have novel targets.
CHAIN is also partnering with Porton Biopharma Ltd and Scitech Engineering Ltd to develop GMP spore manufacturing capability in the UK.
MT: And there is always the question of; will it be of Rx or of Food Supplement status?
We intend to make specific health claims about our product and to have it regulated as a medicine.
We would like to personally thank Dr Green for sharing his aspirations. You may contact CHAIN Biotechnology at https://chainbiotech.com

