Why Phages, and Why Now?
Many modern diseases have been linked to alterations in the microbiome and changing it back could be the key to restoring health. Bacteriophages (phages) are viruses that kill bacteria with high target
specificity (McKenna 2018). Using phages to tweak the microbiome, so that it better resembles that of a healthy person, could improve disease outcomes, and recent faecal transplant research (Zuo et al. 2018) has pointed toward a potential role for phages in the success of these treatments.
While phages are known to be very selective about which bacteria they kill, their use as therapeutics for bacterial infections or as microbiome-tweaking agents has yet to be commercialized in most countries. However, phages are considered to be safe, and billions pass through the human body each day (Barr 2017). Using phages to treat bacterial infections, a process known as phage therapy, has been practiced in countries like Georgia and Poland for decades. Now, in light of the global antibiotic resistance crisis, interest in phage therapy is ramping up in the US and in Western Europe (Gorski et al. 2018).
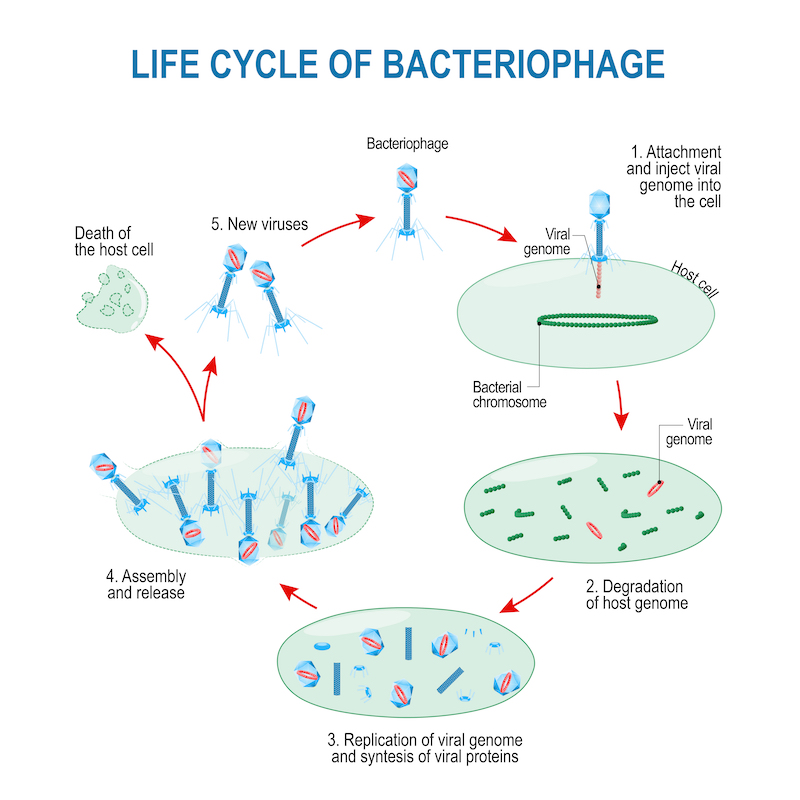
Although phages have been around for a very long time, we haven’t always understood the phages themselves or the problems they could potentially fix. In fact, understanding phages to a degree sufficient to harness their exquisite specificity and diversity, was once a nearly insurmountable challenge. It’s therefore no surprise that we couldn’t readily use phages or assess whether they were working.
Nowadays, however, genome sequencing of phages and entire microbiomes is readily available and reasonably-priced. We now better understand phages and appreciate the important role of microbiomes in health and disease. We also know now that antibiotics aren’t the solution for all bacteria-related illnesses. Because of these shifts, it is becoming easier to imagine how phages could be used to improve our health.
Overcoming the Hurdles to Phage-based Applications
According to researchers who contributed to the special ”Hurdles for Phage Therapy” issue of the journal Viruses, some of the main barriers to phage therapy pertain to the phages themselves (do we know enough about how phages work?), while others pertain to the regulatory and economic environment (will regulatory bodies approve phages, even if they do work, and will companies invest in phages?). It was generally acknowledged that we know a lot about how certain phages act under laboratory conditions, but it is still unclear how most phages work to kill their bacterial targets and in which circumstances.
 Notably, most researchers whose ideas were featured in the Hurdles issue presented possible solutions to these issues, suggesting an overall optimism about the future of phage applications. For instance, one group highlighted the lack
Notably, most researchers whose ideas were featured in the Hurdles issue presented possible solutions to these issues, suggesting an overall optimism about the future of phage applications. For instance, one group highlighted the lack
of standardized criteria for determining a phage’s potential as a safe and effective therapeutic and provided a list of features that make a phage particularly suited for therapy. Desirable features included a genome, free of harmful genes, stability under storage and treatment conditions, anti-biofilm activity, lytic lifestyle and ability to overcome anti-phage systems. In response to a lack of consensus on what should be known about a phage’s genome before it should be used in humans, another group laid out a set of guidelines: identify and annotate all genes using validated computational tools, search for problematic genes (like antibiotic resistance genes, virulence factors and toxins), verify that the genome sequence is representative of the population of phages that were sequenced and make sure to check for contaminating DNA. From a regulatory standpoint, another group detailed how their institution (in Belgium) has worked with regulatory authorities to develop and gain approval for a new phage therapy protocol that gets around the need for traditional clinical trials (Pirnay et al. 2018).
Recent Progress by Phage Companies
To complement the optimism of the academic research community, a handful of American and European companies are now testing phage-based products in human trials. Different companies are using different strategies of phage product development. One strategy is to develop products that work on a large number of patients by combining several phages into “cocktails”. The French company Pherecydes Pharma recently finished testing a phage cocktail strategy in a clinical trial on burn patients (Jault et al. 2018). Unfortunately, this trial showed that phages were less effective than the standard care for burn patients. However, the company admitted that mixing their phages in a cocktail inactivated most of their phages, meaning patients received far fewer phages than was intended. Surprisingly, this low-level phage treatment still did help the patients, suggesting that if high phage titters could be maintained, a future trial might show efficacy.
Similarly, the US company AmpliPhi is also developing phage cocktails and recently presented successful phage treatment of 10/12 patients (AmpliPhi 2018) with life-threatening Staphylococcus aureus bacteremia or sepsis. The company also recently received FDA approval to start phase I/II clinical trials in 2019 using its S. aureus phage cocktail and its Pseudomonas aeruginosa cocktail (AmpliPhi 2018). Another US company, Intralytix, has already commercialized phage cocktail-based products for use in food decontamination and is now moving toward clinical trials for assessing efficacy of phage cocktails against adhesive invasive E. coli (AIEC) infections in inflammatory bowel disease (IBD) patients (Intralyx 2018).
In contrast to developing phage cocktails, another strategy is to test bacteria isolated directly from a patient for susceptibility to a large bank of phages. Then, a custom preparation of phages that specifically kill that patient’s bacterial strain is created and given to the patient. Adaptive Phage Therapeutics (APT) is developing a system of phage therapy in this manner and has already treated several patients on a case-by-case basis (AmpliPhi 2018). This company is planning to begin clinical trials in 2019 (Boodman 2018).
A third strategy by which companies are exploiting phages for therapy is by genetically engineering them to have customized target specificities and killing mechanisms. For example, Locus Biosciences, a company out of North Carolina, is planning E. coli UTI clinical trials for 2019 using engineered phages. As well, the Israeli company BiomX and the French company Eligo Bioscience are also developing engineered phages, though these companies are not yet at testing their products in clinical trials.
Recent Regulatory Shifts Surrounding Phage Applications
In recent years, groups of researchers, industry professionals, and regulatory agency representatives have been gathering to discuss how to develop and commercialize effective phage treatments, such as in the USA (FDA 2017) and Germany (Huber et al. 2018). The general theme emerging is that regulatory bodies are open to the idea of phage therapy, and are urging companies planning phage production to approach them for guidance regarding phage manufacturing and clinical trial development (Huber et al. 2018).
Getting help from regulatory agencies in the early stages of phage product development could save companies significant amounts of time and money. As a cautionary tale, it took Pherecydes Pharma 25 months to manufacture their 12-phage cocktail according to Good Manufacturing Practices (GMP). In the paper they published in October 2018 (Jault et al. 2018), the team described many of the challenges they encountered, including difficulties finding appropriate patients for their trial. Included patients needed to have infections involving only one bacterial species at a time. However, as many wound infections involve the presence of multiple bacterial species, only a small number of patients could be included in their study. This trial is one of the first double-blind, controlled clinical trials done using phages, and represents a wealth of information for any company planning clinical trials with phages. Ideally, companies testing their phage products in human trials can learn both from trials like this and from proactive discussions with regulatory agencies before trials begin.
In part because of what has been learned from trials like Phagoburn, regulatory authorities are now acknowledging (Huber et al. 2018) that the cost and workload associated with manufacturing phages to traditionally appropriate clinical standards can be almost prohibitive for small companies. Some regulatory authorities are now as well recognizing that phages may work better as personalized medicine (Pirnay et al. 2018). Some authorities are even open to the idea of producing phages in a way that does not require GMP manufacturing or traditional clinical trials. For instance, a group of Belgian researchers recently worked with regulatory authorities to successfully set up a new regulatory framework for phage therapy in Belgium (Pirnay et al. 2018). This new framework classifies phages as active pharmaceutical ingredients, not drugs, thus exempting them from clinical trial
requirements and allowing them to be administered by pharmacists on a per- patient basis upon physician prescription.
These examples illustrate how much progress has been made toward surmounting certain hurdles associated with phage therapy. However, other important hurdles still stand in the way of commercializing phage products. For instance, if phages are developed under a personalized medicine model, added intellectual property challenges will need to be faced. As this will undoubtedly discourage investment from companies, particularly those accustomed to dealing with traditional drug development pathways, it is clear that creative ways of incentivizing phage development need to be explored.
Community-driven Efforts to Accelerate Phage Therapy
Some US and European companies, such as AmpliPhi, APT and Pherecydes Pharma, are already treating small numbers of patients each year with phages outside of controlled clinical trials. This provides patients with access to life-saving treatment without having to wait for phage products to get to market. At the same time, it allows companies to get information about if (and when) their products are effective, which can inform more successful clinical trials. However, the resources and time it takes for a company to produce phages on an emergency, case-by-case basis, diverts resources from product development, thus limiting the degree to which companies can participate in this process.
Nonetheless, experimental treatments with phages in this manner have generated fanfare in the US as of recently, particularly after San Diego professor Tom Patterson was successfully treated with phages that targeted his life- threatening bacterial infection (McKenna 2018). In this case, phages were found and prepared as part of a collaborative effort between Patterson’s wife, Dr. Steffanie Strathdee, his doctor, Dr. Robert Schooley, companies like AmpliPhi and APT, and academic labs at Texas A&M and San Diego State University. In the wake of this widely publicized experimental treatment success, physicians and families around the world have reached out to Strathdee and Schooley for phages that could help other patients. In response, the team established the Center for Innovative Phage Applications and Therapeutics or ”IPATH” in June 2018 with the aim of collaborating with companies and academic groups to continue treating patients on an experimental, emergency basis, but also to work towards demonstrating phage efficacy in clinical trials.
Outlook
Overall, the future appears bright for phage-based applications, but it is clear that creative, cross- disciplinary approaches to overcoming commercialization hurdles are needed. It remains to be seen which strategies for commercializing phages will prove effective, but it is clear that momentum is gathering. In light of this progress, regulatory agencies around the world are now beginning to carve out space for phages, companies are beginning to enter the ring and start developing phage products, while community-driven efforts are accelerating tangible changes in the playing field.
About Phage Directory
Phage Directory was created in November 2017 to help systematize efforts toward finding and preparing phages for eligible patients. Phage Directory coordinates labs and companies willing to help prepare phages for patients in need. Phage Directory is expanding its scope and aims to help accelerate the progress of anyone interested in using phages (either medically, academically or commercially) by building and drawing upon a global network of phage researchers, companies and physicians.
References
AmpliPhi (2018), AmpliPhi Biosciences Announces Positive FDA Feedback for its Clinical Stage Bacteriophage Product Candidate AB-PA01 Targeting Pseudomonas Aeruginosa Infections, viewed October 2018, https://investor.ampliphibio.com/press- release/featured/ampliphi-biosciences-announces-positive- fda-feedback-its-clinical-stage-0
Barr, J (2017), ‘A Bacteriophages Journey through the Human Body’, Immunological Reviews, 279 (1): https://onlinelibrary. wiley.com/doi/abs/10.1111/imr.12565#)
Boodman E (2018), ‘How the Navy brought a once-derided scientist out of retirement — and into the virus-selling business’, Statnews, viewed October 2018, https://www. statnews.com/FDA (2017), ‘Transcript of Proceedings: Bacteriophage Therapy: Scientific and Regulatory Therapy Public Workshop’, Heritage Reporting Corporation, viewed October 2018, https://www. fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/ WorkshopsMeetingsConferences/UCM579441.pdf
Gorski A, Międzybrodzki R, Lobocka M, Glowacka-Rutkowska A, Bednarek A, Borysowski J, Jonczyk-Matysiak E, Lusiak- Szelachowska M, Weber-Dabrowska B, Baginska N, Letkiewicz S, Dabrowska K, Scheres J (2018), ‘Phage Therapy: What
Have We Learned?’, Viruses 2018, 10(6), 288: https://doi. org/10.3390/v10060288
Huber I, Potapova K, Kuhn A, Schmidt H, Hinrichs J, Rohde C, Beyer W (2018), ‘1st German Phage Symposium—Conference Report’, Viruses 2018, 10(4): https://doi.org/10.3390/ v10040158
Intralyx (2018), Intralytix Receives FDA clearance to initiate Phase I / IIa clinical trials, viewed October 2018, http://www. intralytix.com/index.php?page=news&id=87
Jault P, Leclerc T, Jennes S, Pirnaey JP, Yok-Ai Q, Resch G, Rousseau AF, Ravat F, Carsin F, Le Floch R, Schaal JV, Soler C, Fevre C, Arnaud I, Bretaudeau L, Gabard J (2018), ‘Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial’, The Lancet Infectious Diseases, viewed October 2018, https:// www.thelancet.com/journals/laninf/article/PIIS1473- 3099(18)30482-1/fulltext
McKenna M (2018), ‘He Was Dying. Antibiotics Weren’t Working. Then Doctors Tried a Forgotten Treatment’, Mother Jones, viewed October 2018, https://www.motherjones.com/ environment/2018/05/the-best-viral-news-youll-ever-read- antibiotic-resistance-phage-therapy-bacteriophage-virus/
Pirnay JP, Verbeken G, Ceyssens PJ, Huys I, De Vos D, Ameloot C, Fauconnier A (2018) ‘The Magistral Phage’, Viruses 2018, 10(2): https://www.mdpi.com/1999-4915/10/2/64/htm
Zuo T, Wong SH, Lam K, Lui R, Cheung K, Tang W, Ching JYL, Chan PKS, Chan MCW, Wu JCY, Chan FKL, Yu J, Sung JJY, Ng
SC (2018), ‘Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome’, Gut, Apr;67(4): https://www.ncbi.nlm. nih.gov/pubmed/28539351

Jessica Sacher
Co-Founder

