The COVID-19 crisis is causing immense suffering and an appalling death toll. It has also dramatically impacted our personal and professional lives, and individuals, businesses and investors can’t wait to get back to normalcy. However, most experts foresee that the state of normalcy we will reach after the worse has passed will actually differ from what we called normalcy before the storm. Changes in (geo)politics, (macro)economy and an accelerated digital transformation are already evident and are likely to stay after the crisis. Within the business world, one of the main aspects all companies will change is their idea of risk and the strategies to mitigate it, as in the current crisis companies capable of maintaining business activity in a remote / digital way are the ones less affected by the lockdowns – some are even amply benefitting. We would like to take a look at how this whole situation is likely to impact the pharma-biotech industry in general, and the microbiome drugs sector in particular.
The entire pharma sector gains momentum
The pharma-biotech sector has been reinforced by the coronavirus crisis so far. Despite the general market volatility and overall bearish behavior, many biotech stocks have reached all-time-highs over the last weeks. Investors, and society as a whole, seem clear that biotechnology is the solution to this healthcare crisis.
This confidence in the healthcare sector has also become evident in authorities, which have shown flexibility with the goal to expedite the approval or availability much needed materials, treatments or vaccines. For instance, on April 17th the European Parliament officially decided to postpone the application of the new Medical Devices Regulation1 to avoid ‘shortages or delays in getting the medical devices needed to fight COVID-19, were they to follow the new rules of the Medical Devices Regulation from May this year’2. In the US, the FDA has created the Coronavirus Treatment Acceleration Program3 which aims at making any COVID-19 treatment available as quickly as possible, and by which the Agency commits to perform ultra-rapid protocol review in only 24 hours. As of April 16, 2020, FDA received 950 inquiries and proposals concerning COVID-19 related drug development. Tens of Fast-Track designations have been already granted for different COVID-19 treatments, vaccines and diagnostics. The FDA is also using the Emergency Use Authorizations (EAU) extensively. The EUA is a procedure by which the FDA Commissioner may allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency when there are no alternatives4. To date, the FDA has issued almost 100 EUA positive letters related to COVID-19.
If this experience turns out to be positive, there is also hope that some facilitating, flexibility measures remain, making the approval of life-saving products easier for the industry. However, some voices are already warning about the perils of pandemic research exceptionalism5 and certain standards are likely to be in fact made stricter in the long term, which may also have an impact on the industry.
Certain biotech key trends accelerate
Something that is becoming evident during this crisis is the importance of mass and early diagnosis in a rapid and cost-efficient way. Countries which deployed efficient testing policies coped with the pandemic less painfully. It is thus to be expected that more attention is paid to diagnostics technologies. Also, long-term efforts to implant mass screening for early detection of certain diseases like some cancers may gain momentum amongst public and private health insurances.
Social distancing and confinement measures, alongside an accelerated digital transformation, are also further pushing the growth of telemedicine as a means to lower the pressure on hospitals and reduce the exposure of lifesaving healthcare professionals to the virus, keeping them safer. Certainly, a trend that will continue to grow in the near future.
Also, one of the greatest mysteries of this pandemic is why the virus kills certain individuals or people in some geographies more than others. A very recent study in fact suggests that up to 50% of the variance in the manifestations of COVID-19 may be due to genetic factors6. Works from the 2002-2003 pandemic suggest that genetic HLA polymorphisms may influence both susceptibility and outcome of SARS7. Given the relationship between HLA polymorphisms in the population and geography, this also opens the question of whether genetic traits are also behind the observed difference of COVID-19 impact in different countries. These investigations may also foster the development of stratified and personalized medicine to better understand risk factors, design containment measures and optimize treatments, not only for COVID-19 but for any other malady.
Infectious diseases sending us a reminder
If there is something humankind is being reminded about is the importance of infectious diseases.
Over the last century, communicable diseases have become a minor cause of death worldwide, especially in developed countries, although there are huge differences between geographies. This has understandably led pharmaceutical companies to focus their efforts on the modern pandemics like cardio-metabolic disease and cancer. Most investors have traditionally kept themselves away from antibiotic and vaccines assets as they tend to be, with very few exceptions, perceived as less profitable investments (high-risk, low-return and long-term). However, in recent years we are receiving multiple warnings about antibiotic resistance as one of the main challenges facing humankind. A report from the WHO in 2019 warned that, if left undealt with, by 2050 multi-drug resistant infections could cause up to 10 million deaths each year worldwide (from the estimated 0.7 million current ones) and cause an ‘economic damage […] comparable to the shocks experienced during the 2008-2009 global financial crisis’8. These words seem premonitory today, even if the cause for the economic meltdown is a virus rather than a bacterium.
Year 2050 seemed like a horizon too long to invest today, and bacteria usually spread less efficiently than viruses, after all. However, these days some reports do highlight that up to 50% of the deaths attributed to coronavirus may have occurred, at least in part, due to secondary bacterial pneumonia9,10. The exact same situation was reported in the 2009 H1N1 influenza pandemic11and a postmortem analysis of casualties from the 1918 flu coincide with this observation12. Is this all a warning of the power of microorganisms?
Microbiome and infectious diseases: useful viruses and more
This general hype about pharma and biotech will certainly also have positive effect on microbiome biotechs. However, there are many factors, starting with an increased awareness of infectious diseases, that will specifically highlight the importance of further exploring the microbiome.
The microbiome is the collective name for the trillions of microbes (bacteria, yeasts, fungi, viruses and bacteriophages) that inhabit our inside (chiefly intestine, but also the lungs, reproductive tract, etc.) and our outside (the skin). Over the last decade, a growing body of research shows that the composition and/or the activity of these microbes are related with human health and disease.
Although microbiome drug biotechs are developing treatments for a wide variety of indications, infectious diseases is the main one, accounting for around a third of all programs currently ongoing. In general, microbiome technologies have the potential to mitigate the antibiotic resistance crisis by either increasing antimicrobial selectivity or acting through mechanisms against which generating resistance is evolutionarily challenging, or both.
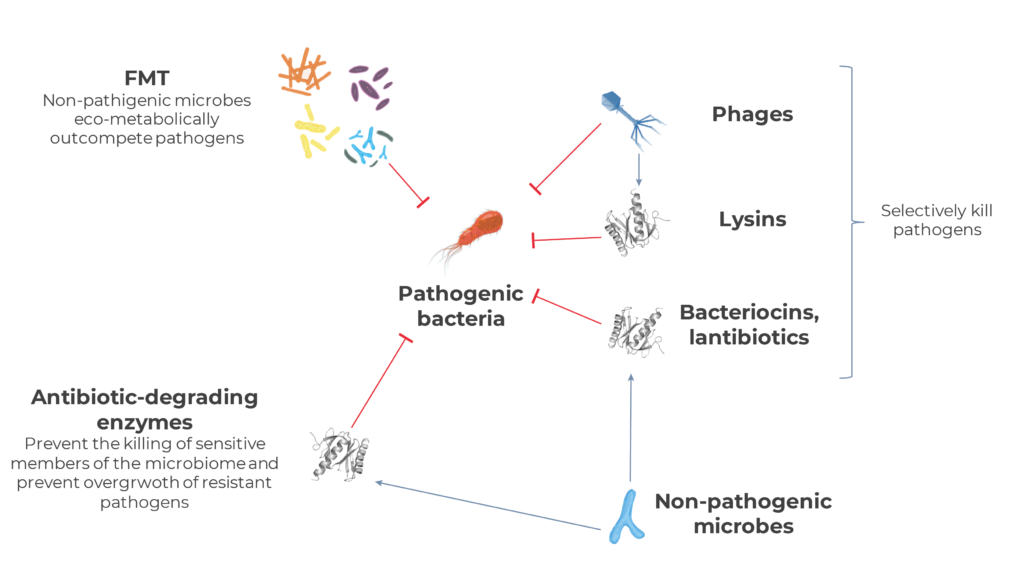
Many programs tackling infectious diseases are focused on Clostridioides difficile infection non responsive to standard therapies. C. difficile infection was one of the first promising application of Fecal Transplant (FMT) in the industry’s earlies days13. C. difficile causes a life-threatening diarrhea and colitis that affects around 225,000 people and kills almost 13,000 each year in the US alone14, and which is developing resistance against the main antibiotics used against it, especially vancomycin15. Multiple trials have shown that FMT can be life-saving when antibiotic therapy fails, by physically and eco-metabolically outcompeting C. difficile rather than aiming at defeating it with chemicals.
Bacteriophages or simply phages are viruses that infect bacteria. Many microbiome companies are mining these bacteria-killing viruses from the microbiome to kill specific antibiotic-resistant microbial species, not only in the gut (e.g. C. difficile, Campylobacter, Salmonella) butalso in different locations such as the bloodstream (e.g. Staphylococcus aureus bacteremia), the airways (e.g. P. aeruginosa infection in patients with cystic fibrosis), the urinary tract (e.g. E. coli) and the skin (P. aeruginosa, S. aureus infecting burns or diabetic ulcers). In many occasions these bacteriophages are engineered to optimize their specificity or effectiveness. Some companies are using parts of phages rather than full viruses. Lysins are enzymes encoded by bacteriophages which allow these viruses either enter (ectolysins) or leave (endolysins) bacterial cells in their infection cycle. In either case, their role is breaking down the surface of the bacterium to allow viral movement. Given their specificity, their activity is being tested as a potential approach for the development of narrow-spectrum antibiotics.
A very common mechanism of antibiotic resistance resides in enzymes capable of degrading antibiotics before they cause harm to the microbe. This same mechanism is being tested against them. In many occasions, diseases caused by antibiotic resistant bacteria happen after a course of antibiotics administered for other purposes. These antibiotics kill off all sensitive bacteria, paving the path for resistant ones to thrive in a half-empty ecosystem. This is the reason why that same antibiotic-degrading enzymes such as beta-lactamases and carbapenemases are being tested to prevent systemically-administered antibiotics from reaching the microbes in the gut. The rationale behind this approach is trying to inactivate those antibiotics coming from the blood into the intestine before they mass-kill sensitive members of the gut microbiome. This way, pathogenic-resistant ones will not have free range to grow and cause damage.
Bacteria also naturally produce peptides they use to inhibit or kill their peer microbes in order to gain competitive advantage in their often-overcrowded habitats. These peptides, such as bacteriocins and lantibiotics, are usually highly genus-specific and thus exhibit narrow killing spectrum. Millenia of evolutionary arms races between bacterial species also make it more difficult to generate resistance against them.
The power of microbes: beyond infectious diseases
The fact that microbes are certainly the cause but also a potential solution to pandemics is also quite reinforcing for the microbiome field in general. Also, the very nature of this biotech niche also seems particularly attractive in the new pharma business world we are headed towards.
Indeed, a recent publication found a strong negative correlation between BCG childhood vaccination and impact of COVID-19 between different countries16. The BCG vaccine contains the live bacterium Mycobacterium bovis (also known as Bacillus Calmette-Guérin, hence the vaccine name) and has been used to immunize against tuberculosis over the last century. Although not confirmed or fully described, it has been suggested that this vaccine may confer protection against coronavirus infection by a mechanism known as trained immunity, also referred to as innate immune memory, by which the innate immune system may be better prepared to fight many or even any infection, as this phenomenon seems to be antigen-nonspecific17. However strange this may sound, the possible protection against virus by certain vaccines not initially designed to create immunity against viruses, as is the case of the BCG vaccine, was known from before18,19and is denominated heterologous (or cross) protection. In fact, the immune-modulating capacity of BCG has been employed to help our immune system fight bladder cancer for years now.
What this example illustrates is how little we still know about the microbiome and thus how much untapped potential and opportunities it still harbors. As in the case of BCG vaccine and coronavirus, we are certainly aware of the importance of the microbiome in the education and the modulation of our immune system. Currently almost one in five microbiome drug development projects are targeted against immune-mediated diseases like Inflammatory Bowel Disease (IBD), rheumatoid arthritis and psoriasis. To these we should add the 100+ active projects ongoing in immuno-oncology, highlighting the applications of microbiome therapies in combination with immune checkpoint inhibitors. Leading big pharmas in this area such as Johnson & Johnson, Takeda, Allergan, Genentech, Abbvie, Merck, Bristol-Myers Squibb, AstraZeneca, Chugai and Astellas have already partnered up with this immune-microbiome startups to thicken their blockbuster portfolios20.
Going back to the coronavirus case, we should not forget that a big component of its virulence is also immune, as the hyper-inflammatory response generated by the human body against the virus, also called cytokine storm syndrome, seems to be responsible for many complications and deaths. Here the immune modulation capacity of the microbiome could also be potentially important, and very recently the microbiome listed company 4D pharma plc (AIM: DDDD) received expedited acceptance from the UK Medicines and Healthcare products Regulatory Agency to commence a Phase II study of its MRx-4DP0004 in patients with COVID-19. MRx-4DP0004, a strain of the bacterium Bifidobacterium breve and originally developed for asthma21, has shown the potential to simultaneously downregulate specific pathological aspects of the hyper-inflammatory response while maintaining the appropriate anti-viral response22. Recent studies further suggest that gut microbiome composition may predict predisposition to severe COVID-19 due to hyper-inflammatory response23.
Outside the classic concept of infectious diseases, the study of the microbiome is also revealing that some diseases regarded today as noncommunicable may actually be transmitted through microbes24,25. Some microbiome biotechs are developing non-conventional diagnostics in which microbiome signatures are used to aid in the diagnosis of IBD, metabolic diseases and cancer. Furthermore, these diagnostics generally require samples such as stool or saliva, which can be obtained by non-invasive equipment and procedures which allow for remote, self-collection, making remote disease management and monitoring in telemedicine easier. Also, studying the microbiome is also revealing that a person’s microbiota may play a crucial role in the way individuals respond to drugs and other treatments26. In order to predict the effect of medicines, we have so far mostly focused on the individual’s own genetic makeup. However, analyzing a patient’s microbiome is a promising strategy to predict how will they respond to certain treatments and hence allow for a significant advance in personalized or stratified medicine. Renewed interest in diagnostics and technological developments in this area are likely to push this segment in microbiome science even further.
Increased awareness about antibiotic resistance is likely to have a twofold effect on microbiome drug developers. On the one hand, as discussed previously, the microbiome offers different potential solutions to deal with this global issue. However, on the other hand, those developing drugs based on live bacteria will probably have to make an extra effort to exclude organisms containing antibiotic resistance genes in their genomes and probably other traits potentially related to deleterious gene transfer (such as presence of prophages or bacteriophage contamination). Changing regulatory standards may also add some additional pressure on ensuring that antibiotic resistance is dealt with more efficiently. This obviously comes on top of the increasingly stringent requirements to exclude the presence of potentially pathogenic bacteria and viruses (like SARS-CoV-2) in microbiome products like FMT27,28.
Last, but not least, although many activities in microbiome drug development obviously require physical steps (such as wet-lab experiments, clinical trials and manufacturing), the youth of the microbiome drugs sector involves that most of these biotechs are in fact mostly virtual, with most of its activities outsourced and not hence physically attached to a particular location. Flexibility and adaptability are clearly an advantage these days and will be increasingly seen as an effective risk-mitigation strategy.
Investors increasingly aware of the power of microbes
Investors also seem to be understanding the potential of the microbiome, as over €3.5Bn in dilutive funding have been secured in the period 2014-2019.
Although 2019 was by far the worst year for microbiome drugs companies in terms of both foundations and investment, in Q1 2020, and despite the challenging situation, 3 new microbiome drug development companies have been founded and one has been acquired by a major pharmaceutical company. Disclosed dilutive investment has reached almost €150M. These clearly contrast with financial year 2019, when only 6 microbiome drug companies were founded and dilutive investment was of some €460M in total. Non-dilutive, public funding has also gone up in 2020 as only 3 grants account for almost €17M. Furthermore, the top-20 listed microbiome therapeutics companies have outperformed the already well-performing NASDAQ Biotech by an average of 15% since the index’s market low on March 16th 2020.
Concluding remarks
We do not really know how exactly the world and the economy will look like after the COVID-19 crisis. What we do know for sure is that many things will change. What is also certain is that the pharmaceutical industry will come out reinforced as virtually the only means to find long-term solutions for situations like the current one, and that the industry, the authorities, funding agencies and investors will pay more attention to previously neglected infectious diseases. Society is getting a reminder of how damaging infectious diseases can be – the (very) hard way. Hopefully these learnings stay and we do not underestimate microorganisms again.
All these factors will likely put the microbiome biotechnology sector under the spotlight as one of the most promising areas within pharmaceuticals and as an investment opportunity but mainly as a way to improve people’s lives and make the world a safer place for all. Despite the probably indirect role of microbiome in coronavirus infection and the current crisis, the microbial dark matter inside and around us does indeed hold the potential to provide us with tools to fight some of the most concerning issues facing humanity, including but not limited to the spread of multidrug-resistant infections.
This threat proved real. Certainly a good moment to remember that most currently approved drugs were originally extracted from natural sources, and that in many occasions that source was a microorganism29. A good moment to remember the power of microbes.
References
1. European Commission. Date of application of the Medical Devices Regulation postponed until May 2021. https://ec.europa.eu/growth/sectors/medical-devices/new-regulations_en (2020).
2. European Parliament. Parliament decides to postpone new requirements for medical devices. https://www.europarl.europa.eu/news/en/press-room/20200415IPR77113/parliament-decides-to-postpone-new-requirements-for-medical-devices (2020).
3. US Food and Drug Administration. Coronavirus Treatment Acceleration Program (CTAP). https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap.
4. US Food and Drug Administration. Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
5. London, A. J. & Kimmelman, J. Against pandemic research exceptionalism. Science (80-. ).eabc1731 (2020) doi:10.1126/science.abc1731.
6. Williams, F. M. K. et al.Self-reported symptoms of covid-19 including symptoms most predictive of SARS-CoV-2 infection, are heritable. medRxiv2020.04.22.20072124 (2020) doi:10.1101/2020.04.22.20072124.
7. Yuan, F. F. et al.Influence of HLA gene polymorphisms on susceptibility and outcome post infection with the SARS-CoV virus. Virol. Sin.29, 128–130 (2014).
8. Interagency Coordination Group on Antimicrobial Resistance. No time to wait: Securing the future from drug-resistant infections. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf (2019).
9. Chen, T. et al.Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ368, m1091 (2020).
10. Zhou, F. et al.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet395, 1054–1062 (2020).
11. Morris, D. E., Cleary, D. W. & Clarke, S. C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol.8, 1041 (2017).
12. Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis.198, 962–970 (2008).
13. van Nood, E. et al.Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med.368, 407–415 (2013).
14. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (2019).
15. Saha, S. et al.Increasing antibiotic resistance in Clostridioides difficile: A systematic review and meta-analysis. Anaerobe58, 35–46 (2019).
16. Miller, A. et al.Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv2020.03.24.20042937 (2020) doi:10.1101/2020.03.24.20042937.
17. Kurthkoti, K. & Das, G. Mechanism of heterologous resistance of BCG to Covid-19. OSF Prepr.(2020) doi:10.31219/osf.io/f32pz.
18. Moorlag, S. J. C. F. M., Arts, R. J. W., van Crevel, R. & Netea, M. G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect.25, 1473–1478 (2019).
19. Angelidou, A. et al.BCG as a case study for precision vaccine development: Lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front. Microbiol.11, 332 (2020).
20. Gosálbez, L. The microbiome biotech landscape: An analysis of the pharmaceutical pipeline. Microbiome Times(2020).
21. Busetti, A. et al.Bifidobacterium breve MRx-4DP0004 protects against airway inflammation in a severe asthma model by suppressing both neutrophil and eosinophil lung infiltration. in (2018).
22. 4D Pharma plc. Clinical update – Phase II COVID-19 study. https://www.4dpharmaplc.com/en/newsroom/press-releases/clinical-update-phase-ii-covid-19-study (2020).
23. Gou, W. et al.Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv2020.04.22.20076091 (2020) doi:10.1101/2020.04.22.20076091.
24. Gosalbez, L. La microbiota humana como estrategia farmacológica en el entorno regulatorio europeo. (2017).
25. Finlay, B. B. Are noncommunicable diseases communicable? Science (80-. ).367, 250 LP – 251 (2020).
26. Pryor, R., Martinez-Martinez, D., Quintaneiro, L. & Cabreiro, F. The role of the microbiome in drug response.Annu. Rev. Pharmacol. Toxicol.60, 417–435 (2020).
27. US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse-events-likely (2020).
28. US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to SARS-CoV-2 and COVID-19. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-additional-safety-protections (2020).
29. Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod.75, 311–335 (2012).

Luis Gosálbez
Sandwalk BioVentures is a specialty strategy, innovation, regulatory and management consulting firm focused on microbiome technologies servicing companies in the food and pharma sectors, as well as financial and strategic investors exploring to enter this field. The company has created the Microbiome Drug Database™, an online repository containing the most extensive and thorough analysis of biotechnology companies developing pharmaceuticals from or through the microbiome.
