Highlights
- Four significant microbiome program milestones expected during 2020, including readouts from two late-stage development programs
- SER-287 Fast Track designation obtained for active mild-to-moderate ulcerative colitis; Phase 2b study enrollment expected to be completed by mid-2020 and top-line data expected in Q3 2020
- SER-109 Phase 3 study design modified; enrollment expected to be completed by the end of 2019 and top-line data now expected in early 2020
“In recent months, Seres has made significant corporate and clinical progress to concentrate our resources on our highest priority therapeutic candidates with the goal of rapidly achieving key pipeline milestones. This has included the appointment of new leadership, pipeline focusing and prioritization, streamlining of costs, initiation of two clinical studies and the initiation of an oncology-focused collaboration with AstraZeneca,”
said Eric D. Shaff, President and Chief Executive Officer at Seres.
“As part of this strategy, we are announcing today the modification of our ongoing SER-109 Phase 3 ECOSPOR III study in patients with recurrent C. difficile infection. We believe this modification meaningfully accelerates the expected timing for top-line data readout while maintaining a high level of scientific and statistical rigor. We remain enthusiastic about the potential for SER-109, which is supported by compelling clinical and mechanistic evidence.”
With our corporate strategy clearly defined, we look forward to a data-rich 2020 with four significant milestones expected: SER-287 Phase 2b readout in mild-to-moderate ulcerative colitis; SER-109 Phase 3 readout in recurrent C. difficile infection; SER-401 Phase 1b readout in metastatic melanoma; and advancing our rationally-designed, fermented SER-301 preclinical program to clinical development for ulcerative colitis. We are also excited to expand the development of new microbiome-based therapeutic approaches for cancer through our recent collaboration with AstraZeneca,” concluded Mr. Shaff.
Program Updates and Corporate Highlights
SER-287 Phase 2b ECO-RESET study in ulcerative colitis: SER-287 is an oral, donor-derived microbiome therapeutic candidate designed to normalize the gastrointestinal microbiome of individuals with ulcerative colitis. In April 2019, Seres obtained U.S. Food & Drug Administration (“FDA”) Fast Track designation for SER-287 for the induction and maintenance of clinical remission of adult subjects with active mild-to-moderate ulcerative colitis. Seres continues to enroll the SER-287 Phase 2b ECO-RESET induction study in patients with active mild-to-moderate ulcerative colitis. The development of SER-287 is supported by a successful Phase 1b study conducted in 58 patients with active mild-to-moderate ulcerative colitis that demonstrated a beneficial impact on clinical remission and endoscopic improvement, various markers of SER-287 biological activity, including SER-287 microbiome engraftment, as well as detection of metabolomic markers and biopsy transcriptional signals correlating with the clinical results. Preliminary data from the study also showed that those patients who achieved clinical remission did not experience a disease flare in the 26-week period following study initiation. In the SER-287 Phase 1b study, the safety profile for SER-287 was comparable with that of placebo with no imbalance of adverse events and no drug-related serious adverse events.
The SER-287 Phase 2b ECO-RESET study was initiated in December 2018 and is expected to enroll approximately 201 patients with mild-to-moderate ulcerative colitis. Based on FDA feedback, Seres expects that with positive Phase 2b study results, the study could serve as one of two pivotal trials to enable a SER-287 Biologics License Application (BLA) submission.
Seres expects to complete enrollment of the SER-287 Phase 2b ECO-RESET study by mid-2020 and report top-line data in the third quarter of 2020.
SER-109 Phase 3 ECOSPOR III study in recurrent C. difficile infection: SER-109 is an oral, donor-derived microbiome therapeutic candidate designed to restore the depleted, or dysbiotic, gastrointestinal microbiome of patients with recurrent C. difficile infection. Seres has been enrolling a 320 patient, placebo-controlled SER-109 Phase 3 study, ECOSPOR III, in patients with recurrent C. difficile infection. All patients enrolled in ECOSPOR III were required to test positive for C. difficilecytotoxin to ensure enrollment of only patients with an active C. difficile infection.
The original 320 patient ECOSPOR III trial was designed to evaluate SER-109 efficacy, a comprehensive safety database, and to serve as a single pivotal study supporting BLA submission. Consistent with the Company’s strategy to obtain rigorous, near-term clinical data, the Company has implemented a revised ECOSPOR III study design that reduces the size of the study to 188 patients. The new size and powering calculations are informed by prior SER-109 study results, published C. difficile infection trial data utilizing cytotoxin testing and preliminary blinded and open label C. difficile infection recurrence rate data from the ongoing ECOSPOR III study. Seres has informed the FDA regarding the ECOSPOR III study modification and plans to further discuss options to expedite the SER-109 development path toward potential BLA submission.
In prior communications with the FDA regarding a potential reduction in ECOSPOR III study size, the agency indicated that if the statistical significance of the outcome of the study is insufficient to support BLA submission, the Company could be required to obtain additional confirmatory evidence of efficacy, such as a second Phase 3 study. Reducing the study size would likely require additional patient exposure to further establish safety. The Company believes that this study revision is designed to provide rigorous efficacy data. Furthermore, based on the safety results observed in all of its microbiome therapeutics clinical trials to date, the Company expects to be able to work with FDA to satisfy additional safety data requirements, if needed.
As of April 30, 2019, ECOSPOR III had enrolled 135 patients. Seres expects to complete enrollment of SER-109 ECOSPOR III by the end of 2019 and report top-line data in early 2020.
SER-401 Phase 1b in metastatic melanoma: SER-401 is an oral microbiome therapeutic candidate comprising a bacterial signature similar to that observed in checkpoint inhibitor immunotherapy responders. The ongoing Phase 1b study, supported by The University of Texas MD Anderson Cancer Center and the Parker Institute for Cancer Immunotherapy, will evaluate the potential for SER-401 to augment response to nivolumab, an approved anti-PD-1 checkpoint inhibitor therapy, and will assess a variety of biological measures of response.
Seres expects to obtain SER-401 Phase 1b preliminary study results in 2020.
SER-301 preclinical candidate: Seres also continues to advance its rationally-designed, fermented microbiome drug discovery and development capabilities. These efforts are focused on advancing SER-301, a preclinical therapeutic candidate for ulcerative colitis, into clinical development. The Company is entitled to a $10 million milestone payment associated with the initiation of SER-301 clinical development from its ongoing collaboration with Nestlé Health Science.
Seres expects to file an Investigational New Drug (IND) application and initiate clinical development for SER-301 in early 2020.
Microbiome immuno-oncology focused collaboration with AstraZeneca: In March 2019, Seres announced a collaboration with MedImmune LLC, a wholly owned subsidiary of AstraZeneca Inc. (“AstraZeneca”) to focus on advancing the mechanistic understanding of the microbiome in augmenting the efficacy of cancer immunotherapy, including potential combination with SER-401. Under the terms of the collaboration, AstraZeneca has agreed to provide Seres with $20 million in three equal installments. In addition, AstraZeneca has agreed to reimburse Seres for research activity related to the collaboration. Seres maintains rights to oncology-targeted microbiome therapeutic candidates, and AstraZeneca has obtained the exclusive option to negotiate for exclusive rights to those programs and other inventions arising out of the collaboration.
Implemented key leadership changes: In January 2019, Seres announced the appointment of Eric D. Shaff as President and Chief Executive Officer. Mr. Shaff, who was Chief Operating and Financial Officer, succeeded Roger J. Pomerantz, M.D., who continues as Chair of Seres’ Board of Directors. Matthew Henn, Ph.D., previously Executive Vice President and Head of Discovery and Microbiome R&D, was appointed Chief Scientific Officer. In February 2019, Seres took action to lower corporate expenses and reduced its full-time workforce by approximately 30%.
Financial Results
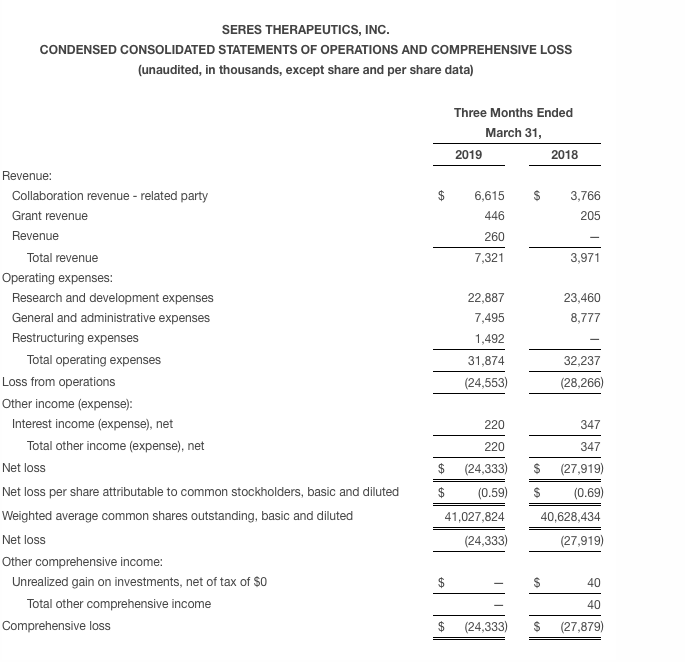
Seres reported a net loss of $24.3 million for the first quarter of 2019, as compared to a net loss of $27.9 million for the same period in 2018. The first quarter net loss was driven primarily by clinical and development expenses, personnel expenses and ongoing development of the Company’s microbiome therapeutics platform. The first quarter net loss figure was inclusive of $7.3 million in recognized revenue associated primarily with the Company’s collaboration with Nestlé Health Science.
Research and development expenses for the first quarter of 2019 were $22.9 million, as compared to $23.5 million for the same period in 2018. The research and development expense was primarily related to Seres’ microbiome therapeutics platform, the clinical development of SER-109 and SER-287, as well as the Company’s immuno-oncology efforts.
General and administrative expenses for the first quarter of 2019 were $7.5 million, as compared to $8.8 million for the same period in 2018. General and administrative expenses were primarily due to headcount, professional fees and facility costs.
During the first quarter of 2019 Seres recognized $1.5 million in restructuring expenses related to the corporate changes discussed earlier.
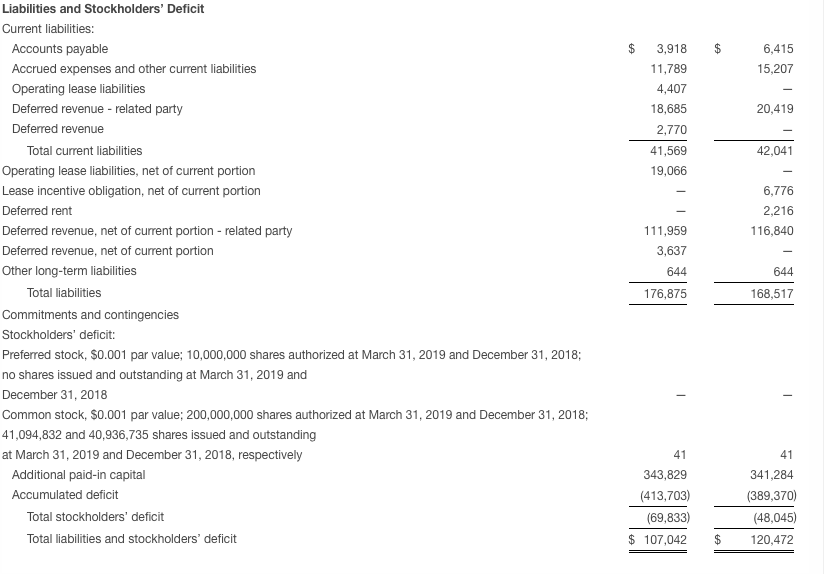
Seres ended the first quarter with approximately $53.6 million in cash and cash equivalents compared with $85.8 million at December 31, 2018. In April 2019 and following the close of the first quarter of 2019, the Company received the first of three $6.7 million annual installment payments due under the terms of the collaboration with AstraZeneca.
Based on the Company’s current operating plan, cash resources are expected to fund operating expenses and capital expenditure requirements, excluding net cash flows from future business development activities or potential incoming milestone payments, into the fourth quarter of 2019.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including the timing and results of each of Seres’ clinical studies, the potential for any of the Company’s studies to serve as a pivotal trial to enable a BLA submission, Seres’ plan to file an IND application for SER-301, the receipt of future milestone payments, the potential impact of any of Seres’ development candidates, the reduction in patient enrollment in the SER-109 Phase 3 trial leading to expedited results, the sufficiency of the Company’s cash resources to fund operating expenses and capital expenditure requirements and other statements that are not historical facts.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have incurred significant losses, are not currently profitable and may never become profitable; our need for additional funding; our limited operating history; our unproven approach to therapeutic intervention; the lengthy, expensive, and uncertain process of clinical drug development; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; the success of our leadership transition; our ability to retain key personnel and to manage our growth; and our management and principal stockholders have the ability to control or significantly influence our business. These and other important factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 6, 2019 and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Source: Seres Therapeutics Inc