This 5th Pharmabiotics Conference Edition, hosted by the Pharmabiotic Research Institute, took place Mid-March in Paris and peaked as the most successful to date, with over 190 delegates attending over the 2-day event.
As in previous editions, Pharmabiotics brought together researchers, big pharma, Contract Research Organizations (CRO) and numerous start-ups. Running parallel with two conference streams, was a one-to-one partnering platform enabling the formation of new collaborations to generate ever more products and science. For this reason this is not an exhaustive report and I apologize to speakers who we were not able to include.
This edition was particularly rich in Biotech introductions, helping to keep an updated view of this quickly evolving landscape.
Personalized Nutrition and Precision Medicine
Joseph Petrosino of Baylor College of Medicine started the event with a presentation on the translation of the Microbiome in the Era of Precision Medicine, envisioning the predictive power of Microbiome analysis in young subjects with regards to risk of diabetes, as well as in order to discriminate responders from non-responders to immunotherapy and antitumour therapy. He mentioned a mouse study in which high fat diet leads to a different microbiome profile which is linked to a sociability defect and decreased levels of oxytocin. It was possible to modulate these responses, but only in a short window of time (in the first 8 weeks), which is consistent with human studies on probiotics and autism (now published data: Liu WY et al., 2019).
Viome’s CSO Momchilo Vuyisich has a vision of the future in which everyone will have their microbiome sequenced to allow for personalized nutrition. However, he highlights that the current microbiome sequencing is insufficient, since the same exact composition can keep somebody healthy and make somebody else sick. The company does RNA transcriptomics, to look at which genes are expressed, a unique approach by function. A super healthy cohort was developed using 30 000 samples and health questionnaires, and the company’s AI is able to predict your health level with your analysis and provide customized nutritional advice to boost missing functions. For example, IBS flares could be prevented by certain foods in certain people, or glycemic peaks…
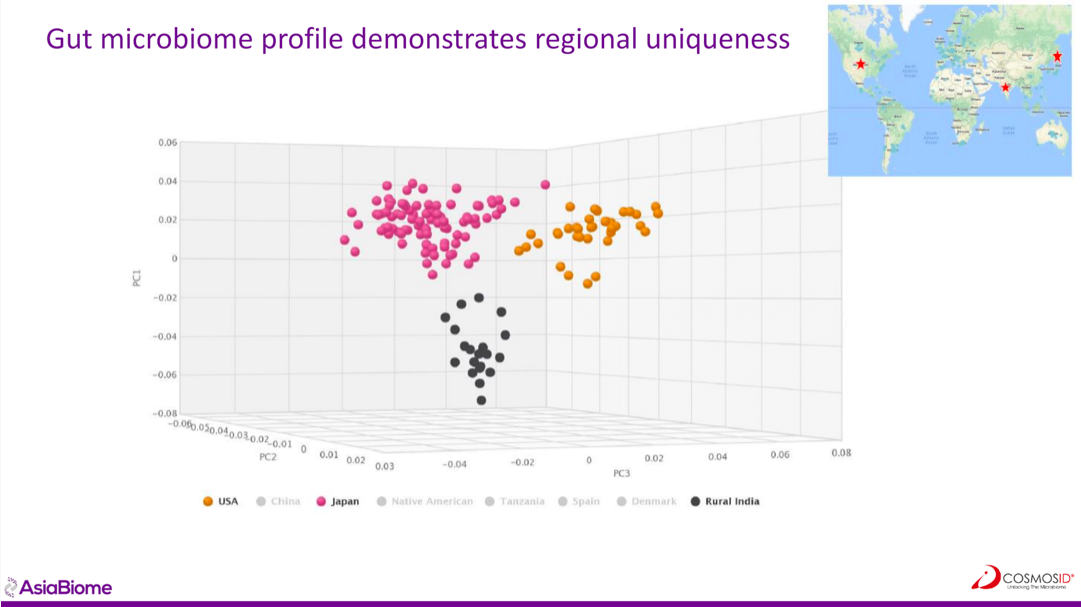 Jonathan Krive, CEO of AsiaBiome, highlighted that among the hundreds of Microbiome companies born in the past 10 years, fewer than 10% look at Asia. Asia represents 60% of the world population, but 60% of bacteria in Asian databases are not present in Western studies, Krive says. Microbiome profiling shows regional uniqueness, which should be taken into account when developing solutions for this target population. So AsiaBiome created the first horizontally integrated microbiome discovery platform in Asia and proposes a microbiota bank as well as a clinical network and an R&D discovery platform to help companies in the field address the Asian specificity and market.
Jonathan Krive, CEO of AsiaBiome, highlighted that among the hundreds of Microbiome companies born in the past 10 years, fewer than 10% look at Asia. Asia represents 60% of the world population, but 60% of bacteria in Asian databases are not present in Western studies, Krive says. Microbiome profiling shows regional uniqueness, which should be taken into account when developing solutions for this target population. So AsiaBiome created the first horizontally integrated microbiome discovery platform in Asia and proposes a microbiota bank as well as a clinical network and an R&D discovery platform to help companies in the field address the Asian specificity and market.
Therapeutic Potential
A. New science helps designing fecal transplant, probiotics and antibiotics:
Olaf Larsen from Yakult / VU University Amsterdam used graph (network) theory to explain the mechanistic rationale behind why Microbiome richness is linked to increased robustness. Fewer interactions and less energy are needed with higher species diversity systems, leading to higher efficiency and resilience within microbial guilds. This information can be a great starting point for a rationally designed fecal transplant analogue, as well as providing an argument for multi-species probiotic preparations.
Irene Wuthrich and Steven Schmitt of Zurich start-up SpheroBiotics have developed a screening tool called nanoFleming, consisting of nanospheres allowing to test millions of bacteria in parallel to find new antibiotics. 74% of antibiotics are derived from microorganisms and 99% of the microbiome was never cultivated, so this platform provides the high throughput necessary to test against pathogens big numbers of microbes from different origins.
B. Skin Microbiome Session
After a talk by Lorenzo Drago on diseases related to the Skin Microbiome, and one by Cédric Bernarde on Metaproteomics and bioinformatics used to decipher microbiota functions, Ingmar Claes of Yun Probiotherapy spoke about the criteria to propose probiotics for topical application on the skin. The product must be safe to use while competing with pathobionts, applicable and functional. Yun developed patented microcapsules able to protect bacteria from the rest of the cream and increase viability and shelf life, to about 6 months. Their formula, based on Lactobacilli inhibiting C. acnesand S. aureus, was tested clinically and showed a significant decrease in pimples and inflammation after 4 weeks.
Ahmad Khodr of L’Oréal described Best Practices for sampling, sequencing and analysis. Indeed, biases can be introduced at each step of the study, and L’Oréal established Standard Operating Procedures (SOP) for skin sampling and accreditation of CROs and genome sequencing platforms, by building mock communities and validating labs that recognize correctly the present bacteria. This allows the company to go with confidence to the next step and test the effect of products topical application. After an aggression to a sensitive skin, the Shannon index of diversity falls and slowly recovers after a few hours. After 7 and 14 days of application of a skin care product, the diversity recovery is quicker. Ahmad tempers the range of application: “deodorants need to use antibacterial properties for their purpose, so it’s not applicable to all products to be bacteria-friendly”.
 The next day, Travis Whitfill from Azitra presented the work of the company in the development of Live Biotherapeutic Products (LBP) for skin disorders (eczema, Netherton syndrome, psoriasis and EGFR-inhibitor associated rash), mostly by engineering Staphylococcus epidermidisto deliver desired protein deep into the skin. Against eczema, AZT-01 addresses skin barrier deficiency by delivering filaggrin, regulates immunity and addresses dysbiosis by inhibiting S. aureus– a concept successfuly tested in mice. To improve Netherton syndrome, recombinant microbes are able to secrete Lekti protein, in optimized dose.
The next day, Travis Whitfill from Azitra presented the work of the company in the development of Live Biotherapeutic Products (LBP) for skin disorders (eczema, Netherton syndrome, psoriasis and EGFR-inhibitor associated rash), mostly by engineering Staphylococcus epidermidisto deliver desired protein deep into the skin. Against eczema, AZT-01 addresses skin barrier deficiency by delivering filaggrin, regulates immunity and addresses dysbiosis by inhibiting S. aureus– a concept successfuly tested in mice. To improve Netherton syndrome, recombinant microbes are able to secrete Lekti protein, in optimized dose.
Georgios Stamatas from Johnson and Johnson mentioned topical products that don’t include bacteria but aim at respecting the skin microbiome and help increase skin microbial richness.
C. Asthma
Siolta Therapeutics was funded in 2016 and CEO Nicole Kimes presented the vision of the company to prevent asthma from birth, by using LBPs to inhibit the immune activation. With 330 million asthma sufferers worldwide and a prevalence of 10-20% in Western countries, increasing by 50% each decade, the world is needing solutions, and Kendall says 3 international cohort studies have shown the concept of prevention of asthma from baby age is valid.
D. The Virome
With over 15 years of experience working on the virome, David Pride from UC San Diego explained the fundamentals of this underlooked community. About 380 trillion viruses are present everywhere in and on our bodies, and they occupy basically any ecosystem. As previously highlighted by Torben Solbeck Rasmussen at Probiota in Copenhagen, a virome transfer can shape your microbiota. Phages appear not to be transient: Abeles et al.showed their persistence over a period of 60 days. As for microbes, different body sites host different types of viromes, which correlate with the bacteria specific to these sites. The saliva and skin present the highest phage diversity, followed by urine and feces. The closest the organs, the more the viromes are similar, and closeness is the factor most contributing to the spread of viruses. In a household, about 25% of viruses are shared, and the virome can grow similar in just 24 hours. Importantly, in some contexts phages do interact with the hosts’s immune system, so they are not necessarily innocuous.
Phage derivates can be used for their specific antimicrobial properties. This is what Paris-based Eligo Bioscience CEO Xavier Duportet explained, with the vision of producing in situ – in hospitals and clinics – selective strain-specific synthetic phage solutions. Testing in mice has shown very good containment in 6 to 72 hours. The team of 27 people is optimizing stability of DNA, improving injection efficiency of the genetic material into the bacteria, and going through in vivo validation assays and regulatory work to define the category and framework for a product that is not a probiotic nor a phage. As well put by Duportet,
“There’s something very cool about hijacking an entity that’s highly evolved since many million years to inject DNA, and the potential of these developments is very exciting in the context of antibioresistance”.
Technology and Methods Creating Science
1.Enumeration of live bacteria: Flow Cytometry
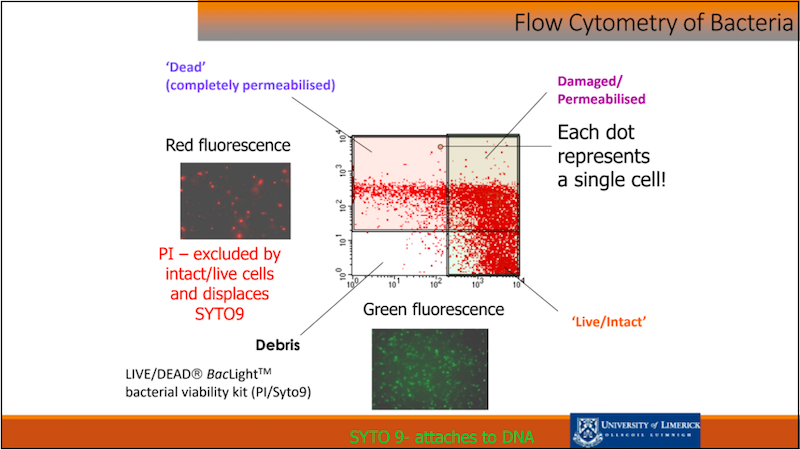 Martin Wilkinson from the University of Limerick gave a fantastic presentation advocating in favour of flow cytometry (FCM) in order to obtain complementary data on your bacteria: FCM catches not only how many live cells are in your samples, but also collects information on their physiology, activity and heterogeneity. Flow cytometry viability data agrees with plate count when samples are predominantly viable freshly grown cultures, when they are highly concentrated like freeze-dried probiotics and when matrix interference is minimal. The Flow cytometry ISO (ISO 19344 IDF 232) found a very good reproducibility and repeatability (except in yogurt due to casein matrix interference, casein being too similar to cells), further improved in Pane et al.,2018.
Martin Wilkinson from the University of Limerick gave a fantastic presentation advocating in favour of flow cytometry (FCM) in order to obtain complementary data on your bacteria: FCM catches not only how many live cells are in your samples, but also collects information on their physiology, activity and heterogeneity. Flow cytometry viability data agrees with plate count when samples are predominantly viable freshly grown cultures, when they are highly concentrated like freeze-dried probiotics and when matrix interference is minimal. The Flow cytometry ISO (ISO 19344 IDF 232) found a very good reproducibility and repeatability (except in yogurt due to casein matrix interference, casein being too similar to cells), further improved in Pane et al.,2018.
Pierre Burguière of AMA Research Solutions / International Dairy Federation prolonged Wilkinson’s ideas on flow cytometry and insisted on the power of this method for R&D, process optimization, quality release and real time performance. Pierre described how combining flow cytometry and specific antibodies allows the specific quantification and viability assessment of each strain in a multispecies product(Chiron et al., 2018), and this under 2 hours. This is a major advantage versus existing methods, and with multiple fields of application. Pierre is now working with ISO and IDF to add this discriminative power in the international standards. It is still time to join the initiative and support the development of antibodies.
2. Metabotype
Marco Pane from Probiotical presented results generated in collaboration with the University of Florence. Nuclear Magnetic Resonance was used to analyse 40 urine samples of 12 individuals, defining for each participant a metabotype, which is actually able to identify an individual with a certainty up to 99% (98% with just 20 samples). At a 10-years follow-up, 9 of the 12 participants were still well-matched while 3 lost their “metabotype identity”. One was healthy but breastfeeding, so her 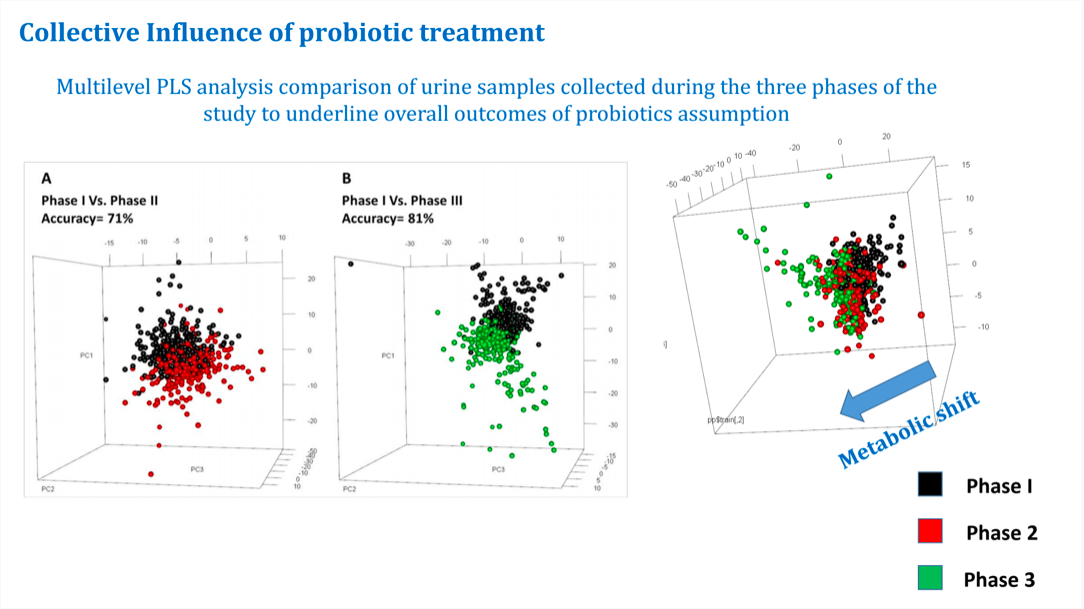 physiological state was changed. One had undergone prolonged antibiotic treatment and developed lactose intolerance, and the third had undergone thyroidectomy, altering deeply the metabolic function fingerprinting. About a year after, the breastfeeding woman and the antibiotic-treated patient had returned to their original metabotype, while the thyroidectomy patient had died of cancer. The research group then tested whether a probiotic supplementation could modify an individual’s metabotype, and indeed the accuracy of prediction dropped from 97-98% pre-intake to 94% after supplementation with B. longum, and down to 89-93% with an association of B. longumand L. delbrueckii. This data supports the vision of the “individual” as a “superorganism”, and confirms the power of probiotics to impact our metabolic identity.
physiological state was changed. One had undergone prolonged antibiotic treatment and developed lactose intolerance, and the third had undergone thyroidectomy, altering deeply the metabolic function fingerprinting. About a year after, the breastfeeding woman and the antibiotic-treated patient had returned to their original metabotype, while the thyroidectomy patient had died of cancer. The research group then tested whether a probiotic supplementation could modify an individual’s metabotype, and indeed the accuracy of prediction dropped from 97-98% pre-intake to 94% after supplementation with B. longum, and down to 89-93% with an association of B. longumand L. delbrueckii. This data supports the vision of the “individual” as a “superorganism”, and confirms the power of probiotics to impact our metabolic identity.
3. OMICS session / Improving Microbiome studies standards
Joel Doré, Research Director at INRA, proposed much awaited and needed standards for Microbiome studies. From the observation that the same sample, analysed by different laboratories, provided huge variability in profiling, the International Human Microbiome Standards (IHMS) set SOPs, adapted per different regulatory contexts, for sample extraction, collection and processing, allowing a very high reproducibility (>98%) when respecting the same set of SOPs. Mock communities are also made available to users from the website. So if you are starting in Microbiome Research, go right here: www.microbiome-standards.org. And do ask experts for support: the longest someone worked on the topic, the best the quality of the data!
Several such expert companies presented their capabilities in microbiome profiling:
Bjorn Nielsen of Clinical Microbiomics described Ultra-High-Resolution Microbiomics, a tool allowing the profiling of microbes and phages with unprecedented resolution, and the possibility to identify Single Nucleotide Variates (SNVs) and small genetic elements including phage-like CAGs and epigenetic information (DNA methylation). This has important implications to increase predictive power in microbiome studies. For example, F. prausnitziiappears to be persistent over time in people, except in the presence of specific phages.
Arne Materna of CosmosID commented on the crucial role of Next Generation Sequencing to identify new therapeutic targets and biomarkers, look at taxonomy down to the strain level (which can mean the difference between life and death!) and to introduce causality (by using function analysis and the host transcriptome). The CRO proposes also whole genome sequencing to look at strain’s safety, preclinical and clinical support, and sequencing depth according to customers needs.
Héloise Breton from DNA Genotek proposed similar services with a more customized approach, solving logistical challenges and looking deeper into your questions to decide upon the depth of sequencing needed.
Finally, Bart Smit from NIZO offered the know-how of the company’s 130 professionals to support culturing and upscaling customer’s LBPs and probiotics, including clinical validation, safety, hints for screening and data interpretation, while tending to leaving you with the Intellectual Property (IP). The group developed an impressive innovation to control the growth of bacteria in consortia, consisting in droplets to seperate each cell in a chamber, leading to independent yet concommitant growth.
Regulatory advice
Frederique Vieville of 5 Quality Business Development Biotech reviewed guidelines and the regulatory framework to support companies start the development of their Investigational Medicinal Products (IMP) on the most solid grounds. Both in EU and in the US, the requirements aim for quality, safety, reliability, efficacy, robustness and batch to batch consistency. This means, in a few words, that equipment needs qualification, methods need validation, personnel needs training and accreditation, raw materials need approval and data has to be well recorded. Importantly, bacteria enumeration must be done with validated methods and comparable across batches, and characterization is needed both on phenotype and genotype, at strain level, and including characterization of the mechanism of action and metabolites.
Yakult’s Bart Degeest explained the work done with the International Probiotics Association (IPA) in the Codex Initiative, supported by Argentina, which is revising the document this year. The scope is the clarification of requirements on characterisation, safety, labelling, though no links to health benefits are planned. The document is expected in approval in July 2020 and guidelines could be effective as of 2023/2024. In the meantime, a paper co-signed by the US Pharmacopoeia was published just this April with technical recommendations to improve the quality of probiotic commercial products with similar focuses, available in open access (Jackson SA et al., Frontiers in Microbiology).
Matthew Steele, Team Leader in the US FDA, provided an excellent frame to understand where your product sits and the steps to get it to market, detailing the authorities expectations. During the development of an Investigational New Drug (IND), as mentioned by Frederique Vieville, Safety, Efficacy and Manufacturing Consistency are the main focuses. The FDA is very transparent and open to guide companies in their process. In fact, Matthew urges Biotechs to talk to the FDA even before Phase 1, as the authorities can orientate the relevance of even the first studies.
 Magali Cordaillat-Simmons gave an overview on regulatory initiatives globally for probiotics and microbiome products. In food supplements, the main initiative is the Codex. In Pharma, both the Pharmabiotics Research Institute (PRI) and the Microbiome Therapeutics Innovation Group (MTIG) are in contact with the European Medical Agency (EMA), but right now the Agency’s responses are slowed down by its moving to Amsterdam. The MTIG is composed of Rebiotix, Seres Therapeutics and Vedanta Biosciences and aims to accelerate Microbiome-based therapies development and market access, in regular contact with the FDA. The group proposes 4 categories for LBPs: donor-sourced microbiome products; defined bacteria consortia; single bacterial strains and genetically-engineered bacteria.
Magali Cordaillat-Simmons gave an overview on regulatory initiatives globally for probiotics and microbiome products. In food supplements, the main initiative is the Codex. In Pharma, both the Pharmabiotics Research Institute (PRI) and the Microbiome Therapeutics Innovation Group (MTIG) are in contact with the European Medical Agency (EMA), but right now the Agency’s responses are slowed down by its moving to Amsterdam. The MTIG is composed of Rebiotix, Seres Therapeutics and Vedanta Biosciences and aims to accelerate Microbiome-based therapies development and market access, in regular contact with the FDA. The group proposes 4 categories for LBPs: donor-sourced microbiome products; defined bacteria consortia; single bacterial strains and genetically-engineered bacteria.
A matter of education
Sophie Durand is one of the co-founders of the French Microbiome Foundation, funded by sponsors, which aims at being at the forefront of a health revolution and raise awareness in the public. The focus has started from digestive diseases and will use medical research findings to help people understand foods that will support a healthy Microbiome and the impacts on health from different angles. The young non-profit organization is now looking for funds and media partnerships.
Investment advice – selecting a Biotech
A panel discussion between Sebastian Guery (DuPont’s leader for Microbiome Ventures), Kristin Wannerberger (same for Ferring) and Aoife Brennan (President and CEO at Synlogic) focused on financing strategies for microbiome Biotechs. The experts agree that the Microbiome is a trend but one that will not go away because this “virtual organ” is deemed to stay with us and will change how we look at health.The field doesn’t see the Return On Investment yet because there are no new drugs on the market right now. With significant trials having started 18-24 months ago, they expect to see more big Pharma in. Kristin is more cautions regarding the timing: “developing new drugs typically takes 10-15 years, but that’s when you have clear guidelines, which we don’t for microbiome based products”. The industry and its research are paving the way to a whole new sector. Sebastian feels developments are accelerating and researchers are getting more used to and comfortable juggling with the complexity of mechanisms. What these investors look for when selecting a Biotech in which to invest comes down to:
- Science and the opportunity to create more knowledge. Clinical trials are an asset;
- Feasibility of taking the product through Phase 1, Phase 2, Phase 3;
- Feasibility of manufacturing;
- Application, and an idea of the market;
- IP;
- Alignment with the company’s strategy, avoiding the dilution of human resources
The panelists expect that companies producing metabolites from probiotics will be the first acquired, because pharma knows how to handle them, while LBPs may take longer to register and bring to market.
You can access the Pharmabiotics 2019 Abstract Book and Presentations via the Conference Website.

Nina Vinot
Before entering industry, Nina was involved in human nutrition research at Penn State University, USA, the French National Center for Scientific Research (CNRS) and the National Institute for Agronomic Research (now INRAe). She has an engineer degree from AgroParisTech, Paris Institute of Technology for Life, Food and Environmental Sciences. After over 6 years developing science-backed probiotic supplements sales across Europe for Probiotical, she is today international sales director for the French biotech TargEDys, the ambassador of precision probiotics.

